Clinician- and Patient-Directed Communication Strategies for Patients With Cancer at High Mortality Risk: A Cluster Randomized Trial
- PMID: 38949813
- PMCID: PMC11217875
- DOI: 10.1001/jamanetworkopen.2024.18639
Clinician- and Patient-Directed Communication Strategies for Patients With Cancer at High Mortality Risk: A Cluster Randomized Trial
Abstract
Importance: Serious illness conversations (SICs) that elicit patients' values, goals, and care preferences reduce anxiety and depression and improve quality of life, but occur infrequently for patients with cancer. Behavioral economic implementation strategies (nudges) directed at clinicians and/or patients may increase SIC completion.
Objective: To test the independent and combined effects of clinician and patient nudges on SIC completion.
Design, setting, and participants: A 2 × 2 factorial, cluster randomized trial was conducted from September 7, 2021, to March 11, 2022, at oncology clinics across 4 hospitals and 6 community sites within a large academic health system in Pennsylvania and New Jersey among 163 medical and gynecologic oncology clinicians and 4450 patients with cancer at high risk of mortality (≥10% risk of 180-day mortality).
Interventions: Clinician clusters and patients were independently randomized to receive usual care vs nudges, resulting in 4 arms: (1) active control, operating for 2 years prior to trial start, consisting of clinician text message reminders to complete SICs for patients at high mortality risk; (2) clinician nudge only, consisting of active control plus weekly peer comparisons of clinician-level SIC completion rates; (3) patient nudge only, consisting of active control plus a preclinic electronic communication designed to prime patients for SICs; and (4) combined clinician and patient nudges.
Main outcomes and measures: The primary outcome was a documented SIC in the electronic health record within 6 months of a participant's first clinic visit after randomization. Analysis was performed on an intent-to-treat basis at the patient level.
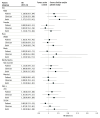
Results: The study accrued 4450 patients (median age, 67 years [IQR, 59-75 years]; 2352 women [52.9%]) seen by 163 clinicians, randomized to active control (n = 1004), clinician nudge (n = 1179), patient nudge (n = 997), or combined nudges (n = 1270). Overall patient-level rates of 6-month SIC completion were 11.2% for the active control arm (112 of 1004), 11.5% for the clinician nudge arm (136 of 1179), 11.5% for the patient nudge arm (115 of 997), and 14.1% for the combined nudge arm (179 of 1270). Compared with active control, the combined nudges were associated with an increase in SIC rates (ratio of hazard ratios [rHR], 1.55 [95% CI, 1.00-2.40]; P = .049), whereas the clinician nudge (HR, 0.95 [95% CI, 0.64-1.41; P = .79) and patient nudge (HR, 0.99 [95% CI, 0.73-1.33]; P = .93) were not.
Conclusions and relevance: In this cluster randomized trial, nudges combining clinician peer comparisons with patient priming questionnaires were associated with a marginal increase in documented SICs compared with an active control. Combining clinician- and patient-directed nudges may help to promote SICs in routine cancer care.
Trial registration: ClinicalTrials.gov Identifier: NCT04867850.
Conflict of interest statement
Figures


References
-
- Wen FH, Chen JS, Su PJ, et al. . Terminally ill cancer patients’ concordance between preferred life-sustaining treatment states in their last six months of life and received life-sustaining treatment states in their last month: an observational study. J Pain Symptom Manage. 2018;56(4):509-518. doi:10.1016/j.jpainsymman.2018.07.003 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

