Preventive Effect of Neuromuscular Training on Chemotherapy-Induced Neuropathy: A Randomized Clinical Trial
- PMID: 38949824
- PMCID: PMC11217888
- DOI: 10.1001/jamainternmed.2024.2354
Preventive Effect of Neuromuscular Training on Chemotherapy-Induced Neuropathy: A Randomized Clinical Trial
Abstract
Importance: Chemotherapy-induced peripheral neuropathy (CIPN) is a highly prevalent and clinically relevant adverse effect of chemotherapy, negatively impacting patient quality of life. The lack of effective preventive or therapeutic options regarding CIPN often requires changes in cancer therapy, potentially resulting in reduced survival.
Objective: To determine whether sensorimotor training (SMT) and whole-body vibration (WBV) training reduce symptoms and decrease the onset of CIPN.
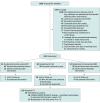
Design, setting, and participants: This prospective multicenter randomized clinical trial (STOP) followed up patients over 5 years at 4 centers in or near Cologne, Germany. Patients undergoing treatment with oxaliplatin or vinca alkaloids were recruited. Participants were recruited from May 2014 to November 2020. Data were last analyzed in June 2021.
Interventions: Participants in the intervention groups performed supervised SMT or WBV training sessions twice a week, each lasting approximately 15 to 30 minutes, concomitant to medical therapy.
Main outcomes and measures: The primary end point was the incidence of CIPN. Secondary end points included subjective neuropathy symptoms, balance control, physical activity levels, quality of life, and clinical outcome. For cross-stratum evaluations, the Mantel-Haenszel test (MH) was used, and within individual strata, Fisher exact test was used for analysis.
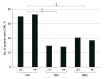
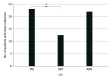
Results: A total of 1605 patients were screened, and 1196 patients did not meet all inclusion criteria, with 251 further excluded or declining participation. A total of 158 patients (mean [SD] age, 49.1 [18.0-82.0] years; 93 [58.9%] male) were randomized into 1 of 3 groups: 55 (34.8%) in SMT, 53 (33.5%) in WBV, and 50 (31.6%) in treatment as usual (TAU). The incidence of CIPN in participants was significantly lower in both intervention groups compared to the control group (TAU): (SMT, 12 of 40 [30.0%; 95% CI, 17.9%-42.1%] and WBV, 14 of 34 [41.2%; 95% CI, 27.9%-54.5%] vs TAU, 24 of 34 [70.6%; 95% CI, 58.0%-83.2%]; P = .002 for intention to treat-MH). Patients receiving vinca alkaloids and performing SMT benefited the most. Results were more pronounced in a per-protocol analysis (>75% participation in the intervention) (SMT, 8 of 28 [28.6%; 95% CI, 16.6%-40.5%] and WBV, 9 of 24 [37.5%; 95% CI, 24.4%-50.5%] vs TAU, 22 of 30 [73.3%; 95% CI, 61.6%-85.6%]). Improvements in favor of SMT compared to TAU were found for balance control bipedal with eyes open; bipedal with eyes closed; monopedal, vibration sensitivity, sense of touch, lower leg strength, pain reduction, burning sensation, chemotherapy dose reductions, and mortality.
Conclusion and relevance: This randomized clinical trial provides initial evidence that neuromuscular training decreases the onset of CIPN.
Trial registration: German Clinical Trials Register: DRKS00006088.
Conflict of interest statement
Figures
Comment on
-
Exercise and Physical Medicine Interventions for Managing Chemotherapy-Induced Peripheral Neuropathy.JAMA Intern Med. 2024 Sep 1;184(9):1053-1055. doi: 10.1001/jamainternmed.2024.2367. JAMA Intern Med. 2024. PMID: 38949805 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

