Oral Insulin Delay of Stage 3 Type 1 Diabetes Revisited in HLA DR4-DQ8 Participants in the TrialNet Oral Insulin Prevention Trial (TN07)
- PMID: 38949847
- PMCID: PMC11362107
- DOI: 10.2337/dc24-0573
Oral Insulin Delay of Stage 3 Type 1 Diabetes Revisited in HLA DR4-DQ8 Participants in the TrialNet Oral Insulin Prevention Trial (TN07)
Abstract
Objective: To explore if oral insulin could delay onset of stage 3 type 1 diabetes (T1D) among patients with stage 1/2 who carry HLA DR4-DQ8 and/or have elevated levels of IA-2 autoantibodies (IA-2As).
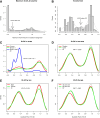
Research and methods: Next-generation targeted sequencing technology was used to genotype eight HLA class II genes (DQA1, DQB1, DRB1, DRB3, DRB4, DRB5, DPA1, and DPB1) in 546 participants in the TrialNet oral insulin preventative trial (TN07). Baseline levels of autoantibodies against insulin (IAA), GAD65 (GADA), and IA-2A were determined prior to treatment assignment. Available clinical and demographic covariables from TN07 were used in this post hoc analysis with the Cox regression model to quantify the preventive efficacy of oral insulin.
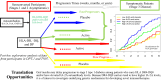
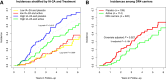
Results: Oral insulin reduced the frequency of T1D onset among participants with elevated IA-2A levels (HR 0.62; P = 0.012) but had no preventive effect among those with low IA-2A levels (HR 1.03; P = 0.91). High IA-2A levels were positively associated with the HLA DR4-DQ8 haplotype (OR 1.63; P = 6.37 × 10-6) and negatively associated with the HLA DR7-containing DRB1*07:01-DRB4*01:01-DQA1*02:01-DQB1*02:02 extended haplotype (OR 0.49; P = 0.037). Among DR4-DQ8 carriers, oral insulin delayed the progression toward stage 3 T1D onset (HR 0.59; P = 0.027), especially if participants also had high IA-2A level (HR 0.50; P = 0.028).
Conclusions: These results suggest the presence of a T1D endotype characterized by HLA DR4-DQ8 and/or elevated IA-2A levels; for those patients with stage 1/2 disease with such an endotype, oral insulin delays the clinical T1D onset.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures
References
-
- Dayan CM, Besser REJ, Oram RA, et al. Preventing type 1 diabetes in childhood. Science 2021;373:506–510 - PubMed
-
- Achenbach P, Barker J, Bonifacio E, Pre-POINT Study Group . Modulating the natural history of type 1 diabetes in children at high genetic risk by mucosal insulin immunization. Curr Diab Rep 2008;8:87–93 - PubMed
-
- Bonifacio E, Ziegler A-G, Klingensmith G, et al.; Pre-POINT Study Group . Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA 2015;313:1541–1549 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

