Regulation of post-translational modification of PD-L1 and associated opportunities for novel small-molecule therapeutics
- PMID: 38949857
- PMCID: PMC11370925
- DOI: 10.1080/17568919.2024.2366146
Regulation of post-translational modification of PD-L1 and associated opportunities for novel small-molecule therapeutics
Abstract
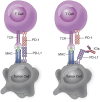
PD-L1 is overexpressed on the surface of tumor cells and binds to PD-1, resulting in tumor immune escape. Therapeutic strategies to target the PD-1/PD-L1 pathway involve blocking the binding. Immune checkpoint inhibitors have limited efficacy against tumors because PD-L1 is also present in the cytoplasm. PD-L1 of post-translational modifications (PTMs) have uncovered numerous mechanisms contributing to carcinogenesis and have identified potential therapeutic targets. Therefore, small molecule inhibitors can block crucial carcinogenic signaling pathways, making them a potential therapeutic option. To better develop small molecule inhibitors, we have summarized the PTMs of PD-L1. This review discusses the regulatory mechanisms of small molecule inhibitors in carcinogenesis and explore their potential applications, proposing a novel approach for tumor immunotherapy based on PD-L1 PTM.
Keywords: N-linked glycosylation; PD-L1; PTMs; acetylation; methylation; palmitoylation; phosphorylation; ubiquitination.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
- 82273792, 82003597/National Natural Science Foundation of China
- B2022201092, B2021201026/Natural Science Foundation of Hebei Province
- S202310075048/Undergraduate Innovation and Entrepreneurship Training Program of Hebei Province
- QN2022057/Natural Science Foundation of Science and Technology Project of Hebei Education Department
- IT2023C01/Innovation Team Project of Hebei University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
