N-Myristoytransferase Inhibition Causes Mitochondrial Iron Overload and Parthanatos in TIM17A-Dependent Aggressive Lung Carcinoma
- PMID: 38949950
- PMCID: PMC11270646
- DOI: 10.1158/2767-9764.CRC-23-0428
N-Myristoytransferase Inhibition Causes Mitochondrial Iron Overload and Parthanatos in TIM17A-Dependent Aggressive Lung Carcinoma
Abstract
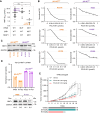
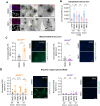
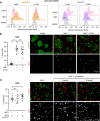
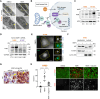
Myristoylation is a type of protein acylation by which the fatty acid myristate is added to the N-terminus of target proteins, a process mediated by N-myristoyltransferases (NMT). Myristoylation is emerging as a promising cancer therapeutic target; however, the molecular determinants of sensitivity to NMT inhibition or the mechanism by which it induces cancer cell death are not completely understood. We report that NMTs are a novel therapeutic target in lung carcinoma cells with LKB1 and/or KEAP1 mutations in a KRAS-mutant background. Inhibition of myristoylation decreases cell viability in vitro and tumor growth in vivo. Inhibition of myristoylation causes mitochondrial ferrous iron overload, oxidative stress, elevated protein poly (ADP)-ribosylation, and death by parthanatos. Furthermore, NMT inhibitors sensitized lung carcinoma cells to platinum-based chemotherapy. Unexpectedly, the mitochondrial transporter translocase of inner mitochondrial membrane 17 homolog A (TIM17A) is a critical target of myristoylation inhibitors in these cells. TIM17A silencing recapitulated the effects of NMT inhibition at inducing mitochondrial ferrous iron overload and parthanatos. Furthermore, sensitivity of lung carcinoma cells to myristoylation inhibition correlated with their dependency on TIM17A. This study reveals the unexpected connection between protein myristoylation, the mitochondrial import machinery, and iron homeostasis. It also uncovers myristoylation inhibitors as novel inducers of parthanatos in cancer, and the novel axis NMT-TIM17A as a potential therapeutic target in highly aggressive lung carcinomas.
Significance: KRAS-mutant lung carcinomas with LKB1 and/or KEAP1 co-mutations have intrinsic therapeutic resistance. We show that these tumors are sensitive to NMT inhibitors, which slow tumor growth in vivo and sensitize cells to platinum-based chemotherapy in vitro. Inhibition of myristoylation causes death by parthanatos and thus has the potential to kill apoptosis and ferroptosis-resistant cancer cells. Our findings warrant investigation of NMT as a therapeutic target in highly aggressive lung carcinomas.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
E. Nemeth is a scientific cofounder of Intrinsic LifeSciences and Silarus Therapeutics and a consultant for Disc Medicine, GKS, Protagonist, Vifor, Ionis, Shield Therapeutics, Novo Nordisk, Chugai, and Chiesi. No disclosures were reported by the other authors.
Figures


References
-
- Giang DK, Cravatt BF. A second mammalian N-myristoyltransferase. J Biol Chem 1998;273:6595–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- TL1 DK132768/DK/NIDDK NIH HHS/United States
- CA208642/GF/NIH HHS/United States
- GM089778/GF/NIH HHS/United States
- UL1TR001881/UCLA CTSI
- Kenneth T. & Eileen L. Norris Foundation
- P30 CA030199/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P30CA030199/GF/NIH HHS/United States
- R35 GM153408/GM/NIGMS NIH HHS/United States
- UCLA JCCC
- R01 CA208642/CA/NCI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- S10 OD023527/OD/NIH HHS/United States
- T32IP5106/UC Regents, TRDRP
- R01 GM089778/GM/NIGMS NIH HHS/United States
- S10OD023527/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

