Use of HSC-targeted LNP to generate a mouse model of lethal α-thalassemia and treatment via lentiviral gene therapy
- PMID: 38949981
- PMCID: PMC11487647
- DOI: 10.1182/blood.2023023349
Use of HSC-targeted LNP to generate a mouse model of lethal α-thalassemia and treatment via lentiviral gene therapy
Abstract
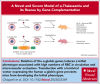
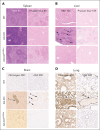
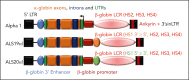
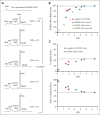
α-Thalassemia (AT) is one of the most commonly occurring inherited hematological diseases. However, few treatments are available, and allogeneic bone marrow transplantation is the only available therapeutic option for patients with severe AT. Research into AT has remained limited because of a lack of adult mouse models, with severe AT typically resulting in in utero lethality. By using a lipid nanoparticle (LNP) targeting the receptor CD117 and delivering a Cre messenger RNA (mRNACreLNPCD117), we were able to delete floxed α-globin genes at high efficiency in hematopoietic stem cells (HSC) ex vivo. These cells were then engrafted in the absence or presence of a novel α-globin-expressing lentiviral vector (ALS20αI). Myeloablated mice infused with mRNACreLNPCD117-treated HSC showed a complete knock out (KO) of α-globin genes. They showed a phenotype characterized by the synthesis of hemoglobin H (HbH; also known as β-tetramers or β4), aberrant erythropoiesis, and abnormal organ morphology, culminating in lethality ∼8 weeks after engraftment. Mice infused with mRNACreLNPCD117-treated HSC with at least 1 copy of ALS20αI survived long term with normalization of erythropoiesis, decreased production of HbH, and amelioration of the abnormal organ morphology. Furthermore, we tested ALS20αI in erythroid progenitors derived from α-globin-KO CD34+ cells and cells isolated from patients with both deletional and nondeletional HbH disease, demonstrating improvement in α-globin/β-globin mRNA ratio and reduction in the formation of HbH by high-performance liquid chromatography. Our results demonstrate the broad applicability of LNP for disease modeling, characterization of a novel mouse model of severe AT, and the efficacy of ALS20αI for treating AT.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: H.P. and D.W. are scientific founders and hold equity in Capstan Therapeutics. Y.K.T. and B.L.M. are employees and hold equity in Acuitas Therapeutics. D.W. receives research support from BioNTech. F.D.B. is founder of Biocept; reports intellectual property licensed to Novartis; and serves as a consultant for Sana Biotechnology, Poseida Therapeutics, Encoded Therapeutics, and Johnson & Johnson. In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, H.P., and D.W. are named on several patents that describe the use of nucleoside-modified mRNA and targeted LNP as platforms to deliver therapeutic proteins and vaccines. The vector ALS20αI is protected in the patent “Generation of a lentiviral vector for the cure of alpha-thalassemia” (The Children's Hospital of Philadelphia, M.E.C., and S.R.). The remaining authors declare no competing financial interests.
Figures



Comment in
-
Genetic medicine gestating for α-thalassemia.Blood. 2024 Oct 10;144(15):1554-1556. doi: 10.1182/blood.2024025938. Blood. 2024. PMID: 39388159 No abstract available.
References
-
- Higgs DR, Gibbons RJ. The molecular basis of alpha-thalassemia: a model for understanding human molecular genetics. Hematol Oncol Clin North Am. 2010;24(6):1033–1054. - PubMed
-
- Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155–167. - PubMed
-
- Galanello R, Cao A. Alpha-thalassemia. Genet Med. 2011;13(2):83–88. - PubMed
-
- Sollaino MC, Paglietti ME, Loi D, Congiu R, Podda R, Galanello R. Homozygous deletion of the major alpha-globin regulatory element (MCS-R2) responsible for a severe case of hemoglobin H disease. Blood. 2010;116(12):2193–2194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

