Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study
- PMID: 38950286
- PMCID: PMC11178534
- DOI: 10.1172/JCI180916
Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study
Abstract
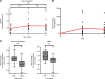
BackgroundRetinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S) is a rare, autosomal dominant, universally fatal disease without effective treatment options. This study explores the safety and preliminary efficacy of crizanlizumab, a humanized monoclonal antibody against P-selectin approved for the prevention of sickle cell crises, in slowing retinal nonperfusion and preserving vision in patients with RVCL-S.METHODSEleven patients with RVCL-S with confirmed exonuclease 3 prime repair exonuclease 1 (TREX1) mutations received monthly crizanlizumab infusions over 2 years. The study measured the nonperfusion index within 3 retinal zones and the total retina with fluorescein angiography, visual acuity, intraocular pressure (IOP), and optical coherence tomography central subfield thickness (CST) at baseline, 1 year, and 2 years. A mixed repeated-measures analysis was performed to assess the progression rates and changes from baseline.RESULTSEleven participants received crizanlizumab infusions. All of the participants tolerated crizanlizumab well, with 8 of 11 (72.7%) reporting mild adverse effects such as nausea, fatigue, and gastrointestinal symptoms. The change in total retinal nonperfusion was 7.22% [4.47, 9.97] in year 1 and -0.69% [-4.06, 2.68] in year 2 (P < 0.001). In the mid periphery, the change in nonperfusion was 10.6% [5.1, 16.1] in year 1 and -0.68% [-3.98, 5.35] in year 2 (P < 0.01), demonstrating a reduction in progression of nonperfusion in the second year of treatment. Visual acuity, IOP, and CST remained stable.CONCLUSIONCrizanlizumab has an acceptable safety profile. These results show promising potential for examining crizanlizumab in larger studies of RVCL-S and similar small-vessel diseases and for using the retina as a biomarker for systemic disease.Trial registrationClinicalTrials.gov NCT04611880.FUNDINGThe Clayco Foundation; DeNardo Education and Research Foundation Grant; Jeffrey T. Fort Innovation Fund; Siteman Retina Research Fund; unrestricted grant from Research to Prevent Blindness Inc.; National Heart,Lung, and Blood Institute (NHLBI), NIH (R01HL129241); National Institute of Neurological Disorders and Stroke (NINDS), NIH (RF1NS116565).
Keywords: Clinical trials; Ophthalmology; Retinopathy.
Conflict of interest statement
Figures






References
-
- de Boer I, et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. GeneReviews. 2019 University of Washington, Seattle, September 19, - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

