Frameshift variants in C10orf71 cause dilated cardiomyopathy in human, mouse, and organoid models
- PMID: 38950288
- PMCID: PMC11178530
- DOI: 10.1172/JCI177172
Frameshift variants in C10orf71 cause dilated cardiomyopathy in human, mouse, and organoid models
Abstract
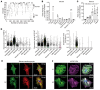
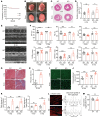
Research advances over the past 30 years have confirmed a critical role for genetics in the etiology of dilated cardiomyopathies (DCMs). However, full knowledge of the genetic architecture of DCM remains incomplete. We identified candidate DCM causal gene, C10orf71, in a large family with 8 patients with DCM by whole-exome sequencing. Four loss-of-function variants of C10orf71 were subsequently identified in an additional group of492 patients with sporadic DCM from 2 independent cohorts. C10orf71 was found to be an intrinsically disordered protein specifically expressed in cardiomyocytes. C10orf71-KO mice had abnormal heart morphogenesis during embryonic development and cardiac dysfunction as adults with altered expression and splicing of contractile cardiac genes. C10orf71-null cardiomyocytes exhibited impaired contractile function with unaffected sarcomere structure. Cardiomyocytes and heart organoids derived from human induced pluripotent stem cells with C10orf71 frameshift variants also had contractile defects with normal electrophysiological activity. A rescue study using a cardiac myosin activator, omecamtiv mecarbil, restored contractile function in C10orf71-KO mice. These data support C10orf71 as a causal gene for DCM by contributing to the contractile function of cardiomyocytes. Mutation-specific pathophysiology may suggest therapeutic targets and more individualized therapy.
Keywords: Cardiology; Cardiovascular disease; Genetic diseases; Genetic variation; Genetics.
Figures





References
-
- Khalil H, Alzahrani T. Cardiomyopathy imaging. In: StatPearls. StatPearls Publishing; 2020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

