Dendritic Cell-Based Immunotherapy in Patients With Resected Pancreatic Cancer
- PMID: 38950309
- PMCID: PMC11379361
- DOI: 10.1200/JCO.23.02585
Dendritic Cell-Based Immunotherapy in Patients With Resected Pancreatic Cancer
Abstract
Purpose: Immunotherapies have shown limited responses in patients with advanced pancreatic cancer. Recently, we reported that dendritic cell (DC)-based immunotherapy induced T-cell responses against pancreatic cancer antigens. The primary objective of this study was to determine the efficacy of DC-based immunotherapy to prevent recurrence of disease.
Methods: This was a single-center, open-label, single-arm, combined phase I/II trial. The primary end point was the 2-year recurrence-free survival (RFS) rate. A 2-year RFS rate of ≥60% was defined as a clinically meaningful improvement. We included patients with pancreatic cancer after resection and completion of standard-of-care (SOC) treatment without recurrent disease on cross-sectional imaging. Patients were treated with autologous DCs pulsed with an allogeneic mesothelioma tumor cell lysate, comprising antigens also expressed in pancreatic ductal adenocarcinoma.
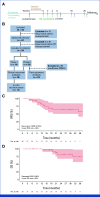
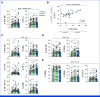
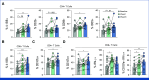
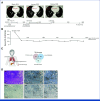
Results: Thirty-eight patients were included in the analysis of the primary end point (47% male, 53% female). The median age was 62 years (IQR, 55-68). Twenty-eight patients (74%) received five DC vaccinations and completed the study protocol. Three patients (8%) received four vaccinations, and seven patients (16%) received three vaccinations. After a median follow-up of 25.5 months, 26 patients (68%) had not developed recurrence of disease. The estimated 2-year RFS was 64%. Vaccination led to the enrichment of circulating activated CD4+ T cells and the detection of treatment-induced immune responses in vitro. T-cell receptor-sequencing analyses of a resected solitary lung metastasis showed influx of vaccine-specific T cells.
Conclusion: This study reached its primary end point of a 2-year RFS rate of ≥60% following pancreatectomy after SOC treatment and adjuvant DC-based immunotherapy in patients with pancreatic cancer. These results warrant a future randomized trial.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Comment in
-
Future of Dendritic Cell-Based Approaches in Pancreatic Cancer.J Clin Oncol. 2024 Sep 10;42(26):3067-3070. doi: 10.1200/JCO.24.00846. Epub 2024 Jul 11. J Clin Oncol. 2024. PMID: 38991191 Free PMC article. No abstract available.
References
-
- Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018 - PubMed
-
- Wainberg ZA, Hochster HS, Kim EJ, et al. : Open-label, phase I study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res 26:4814-4822, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

