CIAO1 loss of function causes a neuromuscular disorder with compromise of nucleocytoplasmic Fe-S enzymes
- PMID: 38950322
- PMCID: PMC11178529
- DOI: 10.1172/JCI179559
CIAO1 loss of function causes a neuromuscular disorder with compromise of nucleocytoplasmic Fe-S enzymes
Abstract
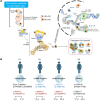
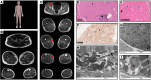
Cytoplasmic and nuclear iron-sulfur (Fe-S) enzymes that are essential for genome maintenance and replication depend on the cytoplasmic Fe-S assembly (CIA) machinery for cluster acquisition. The core of the CIA machinery consists of a complex of CIAO1, MMS19 and FAM96B. The physiological consequences of loss of function in the components of the CIA pathway have thus far remained uncharacterized. Our study revealed that patients with biallelic loss of function in CIAO1 developed proximal and axial muscle weakness, fluctuating creatine kinase elevation, and respiratory insufficiency. In addition, they presented with CNS symptoms including learning difficulties and neurobehavioral comorbidities, along with iron deposition in deep brain nuclei, mild normocytic to macrocytic anemia, and gastrointestinal symptoms. Mutational analysis revealed reduced stability of the variants compared with WT CIAO1. Functional assays demonstrated failure of the variants identified in patients to recruit Fe-S recipient proteins, resulting in compromised activities of DNA helicases, polymerases, and repair enzymes that rely on the CIA complex to acquire their Fe-S cofactors. Lentivirus-mediated restoration of CIAO1 expression reversed all patient-derived cellular abnormalities. Our study identifies CIAO1 as a human disease gene and provides insights into the broader implications of the cytosolic Fe-S assembly pathway in human health and disease.
Keywords: DNA repair; Genetic diseases; Metabolism; Muscle biology.
Figures





Update of
-
Loss of Function of the Cytoplasmic Fe-S Assembly Protein CIAO1 Causes a Neuromuscular Disorder with Compromise of Nucleocytoplasmic Fe-S Enzymes.medRxiv [Preprint]. 2023 Dec 20:2023.12.20.23300170. doi: 10.1101/2023.12.20.23300170. medRxiv. 2023. Update in: J Clin Invest. 2024 Jun 17;134(12):e179559. doi: 10.1172/JCI179559. PMID: 38196629 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

