Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers for Advanced Chronic Kidney Disease : A Systematic Review and Retrospective Individual Participant-Level Meta-analysis of Clinical Trials
- PMID: 38950402
- PMCID: PMC12519637
- DOI: 10.7326/M23-3236
Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers for Advanced Chronic Kidney Disease : A Systematic Review and Retrospective Individual Participant-Level Meta-analysis of Clinical Trials
Abstract
Background: In patients with advanced chronic kidney disease (CKD), the effects of initiating treatment with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB) on the risk for kidney failure with replacement therapy (KFRT) and death remain unclear.
Purpose: To examine the association of ACEi or ARB treatment initiation, relative to a non-ACEi or ARB comparator, with rates of KFRT and death.
Data sources: Ovid Medline and the Chronic Kidney Disease Epidemiology Collaboration Clinical Trials Consortium from 1946 through 31 December 2023.
Study selection: Completed randomized controlled trials testing either an ACEi or an ARB versus a comparator (placebo or antihypertensive drugs other than ACEi or ARB) that included patients with a baseline estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.
Data extraction: The primary outcome was KFRT, and the secondary outcome was death before KFRT. Analyses were done using Cox proportional hazards models according to the intention-to-treat principle. Prespecified subgroup analyses were done according to baseline age (<65 vs. ≥65 years), eGFR (<20 vs. ≥20 mL/min/1.73 m2), albuminuria (urine albumin-creatinine ratio <300 vs. ≥300 mg/g), and history of diabetes.
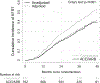
Data synthesis: A total of 1739 participants from 18 trials were included, with a mean age of 54.9 years and mean eGFR of 22.2 mL/min/1.73 m2, of whom 624 (35.9%) developed KFRT and 133 (7.6%) died during a median follow-up of 34 months (IQR, 19 to 40 months). Overall, ACEi or ARB treatment initiation led to lower risk for KFRT (adjusted hazard ratio, 0.66 [95% CI, 0.55 to 0.79]) but not death (hazard ratio, 0.86 [CI, 0.58 to 1.28]). There was no statistically significant interaction between ACEi or ARB treatment and age, eGFR, albuminuria, or diabetes (P for interaction > 0.05 for all).
Limitation: Individual participant-level data for hyperkalemia or acute kidney injury were not available.
Conclusion: Initiation of ACEi or ARB therapy protects against KFRT, but not death, in people with advanced CKD.
Primary funding source: National Institutes of Health. (PROSPERO: CRD42022307589).
Conflict of interest statement
Figures



References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–324. - PubMed
-
- Drawz PE, Beddhu S, Bignall ONR 2nd, Cohen JB, Flynn JT, Ku E, et al. KDOQI US Commentary on the 2021 KDIGO Clinical Practice Guideline for the Management of Blood Pressure in CKD. Am J Kidney Dis. 2022;79(3):311–27. - PubMed
-
- Berger AK, Duval S, Manske C, Vazquez G, Barber C, Miller L, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J. 2007;153(6):1064–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
