The Effects of Vitamin D Supplementation During Pregnancy on Maternal, Neonatal, and Infant Health: A Systematic Review and Meta-analysis
- PMID: 38950419
- PMCID: PMC11819489
- DOI: 10.1093/nutrit/nuae065
The Effects of Vitamin D Supplementation During Pregnancy on Maternal, Neonatal, and Infant Health: A Systematic Review and Meta-analysis
Abstract
Context: Previous research linked vitamin D deficiency in pregnancy to adverse pregnancy outcomes.
Objective: Update a 2017 systematic review and meta-analysis of randomized controlled trials (RCTs) on the effect of vitamin D supplementation during pregnancy, identify sources of heterogeneity between trials, and describe evidence gaps precluding a clinical recommendation.
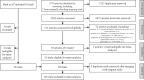
Data sources: The MEDLINE, PubMed, Europe PMC, Scopus, Cochrane Database of Systematic Reviews, Web of Science, and CINAHL databases were searched. Articles were included that reported on RCTs that included pregnant women given vitamin D supplements as compared with placebo, no intervention, or active control (≤600 IU d-1). Risk ratios (RRs) and mean differences were pooled for 38 maternal, birth, and infant outcomes, using random effects models. Subgroup analyses examined effect heterogeneity. The Cochrane risk of bias tool was used.
Data extraction: Included articles reported on a total of 66 trials (n = 17 276 participants).
Data analysis: The median vitamin D supplementation dose was 2000 IU d-1 (range: 400-60 000); 37 trials used placebo. Antenatal vitamin D supplementation had no effect on the risk of preeclampsia (RR, 0.81 [95% CI, 0.43-1.53]; n = 6 trials and 1483 participants), potentially protected against gestational diabetes mellitus (RR, 0.65 [95% CI, 0.49-0.86; n = 12 trials and 1992 participants), and increased infant birth weight by 53 g (95% CI, 16-90; n = 40 trials and 9954 participants). No effect of vitamin D on the risk of preterm birth, small-for-gestational age, or low birth weight infants was found. A total of 25 trials had at least 1 domain at high risk of bias.
Conclusion: Additional studies among the general pregnant population are not needed, given the many existing trials. Instead, high-quality RCTs among populations with low vitamin D status or at greater risk of key outcomes are needed. Benefits of supplementation in pregnancy remain uncertain because current evidence has high heterogeneity, including variation in study context, baseline and achieved end-line 25-hydroxyvitamin D level, and studies with high risk of bias.
Systematic review registration: PROSPERO registration no. CRD42022350057.
Keywords: maternal and child health; meta-analysis; micronutrient supplementation; pregnancy; systematic review; vitamin D.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Life Sciences Institute.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Zhang Y, Gong Y, Xue H, Xiong J, Cheng G.. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125(7):784-793. - PubMed
-
- O'Callaghan KM, Hennessy Á, Hull GL, et al.Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera≥ 25–30 nmol/L: a dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. Am J Clin Nutr. 2018;108(1):77-91. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

