Point-of-Care Testing in Patients with Hereditary Disorders of Primary Hemostasis: A Narrative Review
- PMID: 38950596
- PMCID: PMC12165736
- DOI: 10.1055/s-0044-1787976
Point-of-Care Testing in Patients with Hereditary Disorders of Primary Hemostasis: A Narrative Review
Abstract
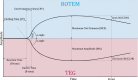
Inherited disorders of primary hemostasis, such as von Willebrand disease and congenital platelet disorders, can cause extensive, typically mucocutaneous bleeding. Assays to diagnose and monitor these disorders, such as von Willebrand factor activity assays and light transmission aggregometry, are performed in specialized hemostasis laboratories but are commonly not available in local hospitals. Due to the complexity and relative scarcity of these conventional assays, point-of-care tests (POCT) might be an attractive alternative in patients with hereditary bleeding disorders. POCTs, such as thromboelastography, are increasingly used to assess hemostasis in patients with acquired hemostatic defects, aiding clinical decision-making in critical situations, such as during surgery or childbirth. In comparison, the use of these assays in patients with hereditary hemostasis defects remains relatively unexplored. This review aims to give an overview of point-of-care hemostasis tests in patients with hereditary disorders of primary hemostasis. A summary of the literature reporting on the performance of currently available and experimental POCTs in these disorders is given, and the potential utility of the assays in various use scenarios is discussed. Altogether, the studies included in this review reveal that several POCTs are capable of identifying and monitoring severe defects in the primary hemostasis, while a POCT that can reliably detect milder defects of primary hemostasis is currently lacking. A better understanding of the strengths and limitations of POCTs in assessing hereditary defects of primary hemostasis is needed, after which these tests may become available for clinical practice, potentially targeting a large group of patients with milder defects of primary hemostasis.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
A.P.B. is an employee of Enzyre BV. W.V.H. is the founder and stockholder of Enzyre BV. Enzyre BV has contracts with Takeda and Novo Nordisk. W.V.H. has received travel support from Takeda for Enzyre-related meetings. S.S. declares no conflict of interest.
Figures




Similar articles
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Platelet Indices and RDW to Assess Inflammatory Milieu in Subclinical Hashimoto's Thyroiditis.Clin Med Insights Endocrinol Diabetes. 2025 Jun 13;18:11795514251349337. doi: 10.1177/11795514251349337. eCollection 2025. Clin Med Insights Endocrinol Diabetes. 2025. PMID: 40528863 Free PMC article.
-
Von Willebrand factor is a multifaceted player in hemostasis requiring a diverse array of analytical and diagnostic approaches.Expert Rev Hematol. 2025 Jul 9:1-18. doi: 10.1080/17474086.2025.2525458. Online ahead of print. Expert Rev Hematol. 2025. PMID: 40555973 Review.
-
Efficacy of regular prophylaxis with a plasma-derived von Willebrand factor/factor VIII concentrate with a 1:1 activity ratio in reducing heavy menstrual bleeding in girls/women with von Willebrand disease.AJOG Glob Rep. 2025 May 2;5(2):100501. doi: 10.1016/j.xagr.2025.100501. eCollection 2025 May. AJOG Glob Rep. 2025. PMID: 40534609 Free PMC article.
References
-
- Bowman M, Hopman W M, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010;8(01):213–216. - PubMed
-
- Oved J H, Lambert M P, Kowalska M A, Poncz M, Karczewski K J. Population based frequency of naturally occurring loss-of-function variants in genes associated with platelet disorders. J Thromb Haemost. 2021;19(01):248–254. - PubMed
-
- Gresele P, Harrison P, Bury L et al. Diagnosis of suspected inherited platelet function disorders: results of a worldwide survey. J Thromb Haemost. 2014;12(09):1562–1569. - PubMed
-
- Pilsczek F H, Rifkin W D, Walerstein S. Overuse of prothrombin and partial thromboplastin coagulation tests in medical inpatients. Heart Lung. 2005;34(06):402–405. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

