Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training
- PMID: 38950777
- PMCID: PMC11294728
- DOI: 10.1016/j.molmet.2024.101980
Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training
Abstract
Objective: In this investigation, we addressed the contribution of the core circadian clock factor, BMAL1, in skeletal muscle to both acute transcriptional responses to exercise and transcriptional remodeling in response to exercise training. Additionally, we adopted a systems biology approach to investigate how loss of skeletal muscle BMAL1 altered peripheral tissue homeostasis as well as exercise training adaptations in iWAT, liver, heart, and lung of male mice.
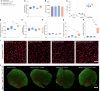
Methods: Combining inducible skeletal muscle specific BMAL1 knockout mice, physiological testing and standardized exercise protocols, we performed a multi-omic analysis (transcriptomics, chromatin accessibility and metabolomics) to explore loss of muscle BMAL1 on muscle and peripheral tissue responses to exercise.
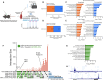
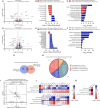
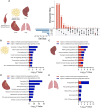
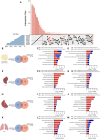
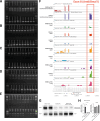
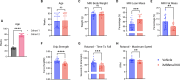
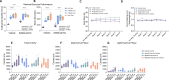
Results: Muscle-specific BMAL1 knockout mice demonstrated a blunted transcriptional response to acute exercise, characterized by the lack of upregulation of well-established exercise responsive transcription factors including Nr4a3 and Ppargc1a. Six weeks of exercise training in muscle-specific BMAL1 knockout mice induced significantly greater and divergent transcriptomic and metabolomic changes in muscle. Surprisingly, liver, lung, inguinal white adipose and heart showed divergent exercise training transcriptomes with less than 5% of 'exercise-training' responsive genes shared for each tissue between genotypes.
Conclusions: Our investigation has uncovered the critical role that BMAL1 plays in skeletal muscle as a key regulator of gene expression programs for both acute exercise and training adaptations. In addition, our work has uncovered the significant impact that altered exercise response in muscle and its likely impact on the system plays in the peripheral tissue adaptations to exercise training. Our work also demonstrates that if the muscle adaptations diverge to a more maladaptive state this is linked to increased gene expression signatures of inflammation across many tissues. Understanding the molecular targets and pathways contributing to health vs. maladaptive exercise adaptations will be critical for the next stage of therapeutic design for exercise mimetics.
Keywords: Circadian biology; Exercise; Inflammation; Metabolism; Signal transduction; Transcription.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures









Update of
-
Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training.bioRxiv [Preprint]. 2024 Apr 27:2023.10.13.562100. doi: 10.1101/2023.10.13.562100. bioRxiv. 2024. Update in: Mol Metab. 2024 Aug;86:101980. doi: 10.1016/j.molmet.2024.101980. PMID: 37905004 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

