Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma
- PMID: 38950910
- PMCID: PMC11671994
- DOI: 10.1136/gutjnl-2024-332398
Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma
Abstract
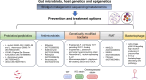
Metabolic dysfunction-associated steatotic liver disease (MASLD) encompasses a wide spectrum of liver injuries, ranging from hepatic steatosis, metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, cirrhosis to MASLD-associated hepatocellular carcinoma (MASLD-HCC). Recent studies have highlighted the bidirectional impacts between host genetics/epigenetics and the gut microbial community. Host genetics influence the composition of gut microbiome, while the gut microbiota and their derived metabolites can induce host epigenetic modifications to affect the development of MASLD. The exploration of the intricate relationship between the gut microbiome and the genetic/epigenetic makeup of the host is anticipated to yield promising avenues for therapeutic interventions targeting MASLD and its associated conditions. In this review, we summarise the effects of gut microbiome, host genetics and epigenetic alterations in MASLD and MASLD-HCC. We further discuss research findings demonstrating the bidirectional impacts between gut microbiome and host genetics/epigenetics, emphasising the significance of this interconnection in MASLD prevention and treatment.
Keywords: FATTY LIVER; GENE EXPRESSION; INTESTINAL MICROBIOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
