Infant formula supplemented with milk fat globule membrane compared with standard infant formula for the cognitive development of healthy term-born formula-fed infants: protocol for a randomised controlled trial
- PMID: 38951000
- PMCID: PMC11331355
- DOI: 10.1136/bmjopen-2023-083399
Infant formula supplemented with milk fat globule membrane compared with standard infant formula for the cognitive development of healthy term-born formula-fed infants: protocol for a randomised controlled trial
Abstract
Introduction: Milk fat globule membrane (MFGM) is a complex lipid-protein structure in mammalian milk and human milk that is largely absent from breastmilk substitutes. The objective of this trial is to investigate whether providing infant formula enriched with MFGM versus standard infant formula improves cognitive development at 12 months of age in exclusively formula-fed full-term infants.
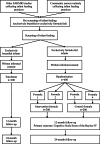
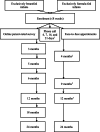
Methods and analysis: This is a randomised, controlled, clinician-blinded, researcher-blinded and participant-blinded trial of two parallel formula-fed groups and a breastfed reference group that were recruited in the suburban Adelaide (Australia) community by a single study centre (a medical research institute). Healthy, exclusively formula-fed, singleton, term-born infants under 8 weeks of age were randomised to either an MFGM-supplemented formula (intervention) or standard infant formula (control) from enrolment until 12 months of age. The reference group was not provided with formula. The primary outcome is the Cognitive Scale of the Bayley Scales of Infant Development, Fourth Edition (Bayley-IV) at 12 months. Secondary outcomes are the Bayley-IV Cognitive Scale at 24 months, other Bayley-IV domains (language, motor, emotional and behavioural development) at 12 and 24 months of age, infant attention at 4 and 9 months of age, parent-rated language at 12 and 24 months of age, parent-rated development at 6 and 18 months of age as well as growth, tolerance and safety of the study formula. To ensure at least 80% power to detect a 5-point difference in the mean Bayley-IV cognitive score, >200 infants were recruited in each group.
Ethics and dissemination: The Women's and Children Health Network Human Research Ethics Committee reviewed and approved the study (HREC/19/WCHN/140). Caregivers gave written informed consent prior to enrolling in the trial. Findings of this study will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number: ACTRN12620000552987; Australian and New Zealand Clinical Trial Registry: anzctr.org.au.
Keywords: Developmental neurology & neurodisability; NUTRITION & DIETETICS; PAEDIATRICS; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The project was funded by the sponsor, Fonterra Co-Operative Group Limited, Auckland, New Zealand, and funding was paid to the South Australian Health and Medical Research Institute. MM is supported by an Australian National Health and Medical Research Council Principal Research Fellowship ID: GNT 2016756. Study products were provided by Fonterra Co-Operative Group Limited. The Australian National Health and Medical Research Council has no role in the study design; collection, management, analysis and interpretation of data; writing of the report; and the decision to submit the report for publication and have no authority over any of these activities. Honoraria have been paid to Dr Gould’s institution to support conference travel by NuMega Ingredients. No other competing interests are declared.
Figures
References
-
- Horta B, CG V. Long-Term Effects of Breastfeeding: A Systematic Review. Geneva, Switzerland, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
