Delayed tumor-draining lymph node irradiation preserves the efficacy of combined radiotherapy and immune checkpoint blockade in models of metastatic disease
- PMID: 38951172
- PMCID: PMC11217506
- DOI: 10.1038/s41467-024-49873-y
Delayed tumor-draining lymph node irradiation preserves the efficacy of combined radiotherapy and immune checkpoint blockade in models of metastatic disease
Abstract
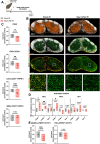
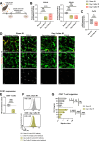
Cancer resistance to immune checkpoint inhibitors motivated investigations into leveraging the immunostimulatory properties of radiotherapy to overcome immune evasion and to improve treatment response. However, clinical benefits of radiotherapy-immunotherapy combinations have been modest. Routine concomitant tumor-draining lymph node irradiation (DLN IR) might be the culprit. As crucial sites for generating anti-tumor immunity, DLNs are indispensable for the in situ vaccination effect of radiotherapy. Simultaneously, DLN sparing is often not feasible due to metastatic spread. Using murine models of metastatic disease in female mice, here we demonstrate that delayed (adjuvant), but not neoadjuvant, DLN IR overcomes the detrimental effect of concomitant DLN IR on the efficacy of radio-immunotherapy. Moreover, we identify IR-induced disruption of the CCR7-CCL19/CCL21 homing axis as a key mechanism for the detrimental effect of DLN IR. Our study proposes delayed DLN IR as a strategy to maximize the efficacy of radio-immunotherapy across different tumor types and disease stages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

