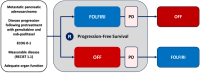
Sequential therapy of refractory metastatic pancreatic cancer with 5-FU/LV/irinotecan (FOLFIRI) vs. 5-FU/LV/oxaliplatin (OFF). The PANTHEON trial (AIO PAK 0116)
- PMID: 38951245
- PMCID: PMC11217046
- DOI: 10.1007/s00432-024-05827-x
Sequential therapy of refractory metastatic pancreatic cancer with 5-FU/LV/irinotecan (FOLFIRI) vs. 5-FU/LV/oxaliplatin (OFF). The PANTHEON trial (AIO PAK 0116)
Abstract
Purpose: In patients with metastatic pancreatic cancer, after failure of gemcitabine/nab-paclitaxel, this trial compares the efficacy of second-line therapy with FOLFIRI vs. OFF (1:1 randomisation) with cross-over to the vice-versa regimen as third-line therapy.
Patients and methods: The primary endpoint was PFS (progression-free survival: time from randomization until progression or death) of second-line therapy. The trial aimed to demonstrate non-inferiority of FOLFIRI vs OFF (non-inferiority margin of a hazard ratio (HR) of 1.5, power of 80% and a significance level of 5%, 196 events needed). Secondary endpoints included overall survival (OS), progression-free survival of third-line therapy and safety. The trial is registered with EudraCT Nr. 2016-004640-11.
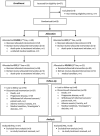
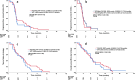
Results: The trial was terminated with 60 evaluable (37 with FOLFIRI, 23 with OFF) patients due to insufficient recruitment. PFS of second-line therapy was 2.4 (95% CI 2.3-2.6) months with FOLFIRI vs 2.4 (95% CI 2.2-2.7) months with OFF (HR: 0.80, 95% CI 0.45-1.42, P = 0.43). OS was comparable between the arms (HR: 0.95, 95% CI 0.54-1.66), P = 0.84). Only 4 out of 28 (14%) patients receiving third-line therapy achieved a disease control (partial remission or stable disease). Both second-line regimens were well tolerated without new or unexpected safety signals being observed.
Conclusion: The exploratory analysis of this early terminated trial suggests that FOLFIRI and OFF have similar efficacy ant toxicity as second-line therapy of PDAC after failure of gemcitabine/nab-paclitaxel. Third-line therapy regardless of regimen does not provide satisfactory efficacy in this sequential treatment algorithm.
Keywords: Chemotherapy; FOLFIRI; Fluoropyrimidine; Metastatic; Oxaliplatin; Pancreatic cancer.
© 2024. The Author(s).
Conflict of interest statement
UG: honoraria/Advisory role: Boehringer INgelheim, AstraZeneca, Bristol-Myers-Squibb, MSD, Sanofi, Fujifilm, Novartis, Amgen. Travel support: GSK, Boehringer-Ingelheim, Amgen, Merck KGaA AHSA: Honoraria: MSD, Merck CD: Honoraria: Abbvie, Bayer, Celgene, Incyte, Janssen, Novartis HO: research funding: Bristol, Servier SS: honoraria: Amgen, AstraZeneca, Bayer, BMS, ESAI, Leo Pharma, Lilly, Merck, MSD, PierreFabre, Roche, Sanofi, Servier, Taiho, Takeda. Research funding: Merck, Pierre Fabre, Servier, Roche, MSD, Novartis, Biontech, ISofol, Mirati, Amgen AK: Honoraria: Merck AS: Honoraria: Roche, Servier, Taiho, Amgen, Pfizer, Lilly, Merck OW: honoraria: Amgen, Bayer, BMS, Celgene, Eisai, Incyte, Ipsen, MerckSerono, MSD, Novartis, Roche, Servier, AstraZeneca. Travel support: Abbvie, Bayer, BMS, Gilead, Ipsen, Medac, Merck. research funding (inst): Merck VH: Honoraria: Merck, Amgen, Roche, Sanofi, Sirtex, Servier, Pfizer, Pierre-Fabre, AstraZeneca, BMS; MSD, Novartis, Boehringer-INgelheim, Celgene, Terumo, Oncosil, Seagen. Support to institution: Merck, Amgen, Roche, Sanofi, Pfizer, Boeringer-Ingelheim, Sirtex, Servier DPM: Honoraria: Merck, Amgen, Servier, AstraZenenca, MSD, Sanofi, Lilly, Pierre-Fabre, Seagen, Onkowissen, IKF, MCI-Deutschland, Jörg Eickeler Veranstaltungen, Taiho, Takeda, Medscape, Cor2ED, G1, 21up, FOMF, Aptitude, GSK. Travel support: Merck, Amgen, Servier. Research funding (inst): Servier, Amgen
Figures
References
-
- Bennouna J, Sastre J, Arnold D et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14(1):29–37 - PubMed
-
- Carrato A, Pazo-Cid R, Macarulla T et al (2022) Sequential nab-paclitaxel/gemcitabine followed by modified FOLFOX for first-line metastatic pancreatic cancer The SEQUENCE trial. J Clin Oncol 40:4022
-
- Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825 - PubMed
-
- Conroy T, Hammel P, Hebbar M et al (2018) FOLFIRINOX or Gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379(25):2395–2406 - PubMed
-
- Ducreux M, Cuhna AS, Caramella C et al (2015) Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v56-68 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

