The OCT2/MATE1 Interaction Between Trifluridine, Metformin and Cimetidine: A Crossover Pharmacokinetic Study
- PMID: 38951433
- PMCID: PMC11271341
- DOI: 10.1007/s40262-024-01390-3
The OCT2/MATE1 Interaction Between Trifluridine, Metformin and Cimetidine: A Crossover Pharmacokinetic Study
Abstract
Background and objectives: Trifluridine/tipiracil, registered for the treatment of patients with metastatic gastric and colorectal cancer, is a substrate and inhibitor for the organic cation transporter 2 (OCT2) and the multidrug and toxin extrusion protein 1 (MATE1), which raises the potential for drug-drug interactions with other OCT2/MATE1 modulators. Therefore, we prospectively examined the effect of an OCT2/MATE1 inhibitor (cimetidine) and substrate (metformin) on the pharmacokinetics of trifluridine.
Methods: In this three-phase crossover study, patients with metastatic colorectal or gastric cancer were sequentially treated with trifluridine/tipiracil alone (phase A), trifluridine/tipiracil concomitant with metformin (phase B) and trifluridine/tipiracil concomitant with cimetidine (phase C). The primary endpoint was the relative difference in exposure of trifluridine assessed by the area under the curve from timepoint zero to infinity. A > 30% change in exposure was considered clinically relevant. A p-value of < 0.025 was considered significant because of a Bonferroni correction.
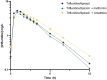
Results: Eighteen patients were included in the analysis. Metformin did not significantly alter the exposure to trifluridine (- 12.6%; 97.5% confidence interval - 25.0, 1.8; p = 0.045). Cimetidine did alter the exposure to trifluridine significantly (+ 18.0%; 97.5% confidence interval 4.5, 33.3; p = 0.004), but this increase did not meet our threshold for clinical relevance. Metformin trough concentrations were not influenced by trifluridine/tipiracil.
Conclusions: Our result suggests that the OCT2/MATE1 modulators cimetidine and metformin can be co-administered with trifluridine/tipiracil without clinically relevant effects on drug exposure.
Clinical trial registration: NL8067 (registered 04-10-2019).
© 2024. The Author(s).
Conflict of interest statement
Bianca Mostert received consulting fees from Lilly, Servier, BMS, AstraZeneca and Amgen, and research funding from Sanofi, Pfizer and BMS. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48. 10.1016/S1470-2045(18)30739-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

