Low-intensity pulsed ultrasound stimulation (LIPUS) modulates microglial activation following intracortical microelectrode implantation
- PMID: 38951525
- PMCID: PMC11217463
- DOI: 10.1038/s41467-024-49709-9
Low-intensity pulsed ultrasound stimulation (LIPUS) modulates microglial activation following intracortical microelectrode implantation
Erratum in
-
Author Correction: Low-intensity pulsed ultrasound stimulation (LIPUS) modulates microglial activation following intracortical microelectrode implantation.Nat Commun. 2024 Sep 2;15(1):7618. doi: 10.1038/s41467-024-52088-w. Nat Commun. 2024. PMID: 39223163 Free PMC article. No abstract available.
Abstract
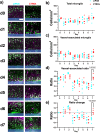
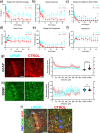
Microglia are important players in surveillance and repair of the brain. Implanting an electrode into the cortex activates microglia, produces an inflammatory cascade, triggers the foreign body response, and opens the blood-brain barrier. These changes can impede intracortical brain-computer interfaces performance. Using two-photon imaging of implanted microelectrodes, we test the hypothesis that low-intensity pulsed ultrasound stimulation can reduce microglia-mediated neuroinflammation following the implantation of microelectrodes. In the first week of treatment, we found that low-intensity pulsed ultrasound stimulation increased microglia migration speed by 128%, enhanced microglia expansion area by 109%, and a reduction in microglial activation by 17%, indicating improved tissue healing and surveillance. Microglial coverage of the microelectrode was reduced by 50% and astrocytic scarring by 36% resulting in an increase in recording performance at chronic time. The data indicate that low-intensity pulsed ultrasound stimulation helps reduce the foreign body response around chronic intracortical microelectrodes.
© 2024. The Author(s).
Conflict of interest statement
J.I. Broussard, N.N. Tirko, J. Greaser, K.W. Gheres, R. Bagwell, and M. Mulvihill have financial stakes in Actuated Medical. Actuated Medical has a patent pending on the hardware design and ultrasound stimulus technology. The inventors are Alanoud S. Alsubhi, Roger B. Bagwell, Jenna K. Greaser, Kevin A. Snook, Natasha A. Tirko, Ryan Clement, and Kyle Gheres. Application # is: 17/837,766. However, it is important to note that the competing interests did not affect the design, methodology, or interpretation of the results in any way. We declare this competing interest to maintain transparency and to provide readers with full disclosure. The remaining authors declare no competing interests.
Figures
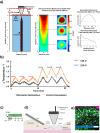
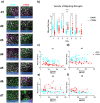
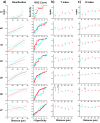
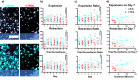


Update of
-
Low-intensity pulsed ultrasound stimulation (LIPUS) modulates microglial activation following intracortical microelectrode implantation.bioRxiv [Preprint]. 2023 Dec 7:2023.12.05.570162. doi: 10.1101/2023.12.05.570162. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 29;15(1):5512. doi: 10.1038/s41467-024-49709-9. PMID: 38105969 Free PMC article. Updated. Preprint.
References
-
- Tarantini, S., Tran, C. H. T., Gordon, G. R., Ungvari, Z. & Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol.94, 52–58 (2017). 10.1016/j.exger.2016.11.004 - DOI - PMC - PubMed
MeSH terms
Grants and funding
- R01 NS105691/NS/NINDS NIH HHS/United States
- R01NS129632/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R21 EB028055/EB/NIBIB NIH HHS/United States
- R01NS115707/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- CAREER 1943906/NSF | ENG/OAD | Division of Chemical, Bioengineering, Environmental, and Transport Systems (CBET)
- R01 NS094396/NS/NINDS NIH HHS/United States
- R21EB028055/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R44 MH131514/MH/NIMH NIH HHS/United States
- R44MH131514/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS129632/NS/NINDS NIH HHS/United States
- R01NS094396/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01NS105691/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS115707/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

