Study on the Infrared and Raman spectra of Ti3AlB2, Zr3AlB2, Hf3AlB2, and Ta3AlB2 by first-principles calculations
- PMID: 38951592
- PMCID: PMC11217358
- DOI: 10.1038/s41598-024-65980-8
Study on the Infrared and Raman spectra of Ti3AlB2, Zr3AlB2, Hf3AlB2, and Ta3AlB2 by first-principles calculations
Abstract
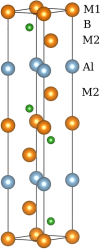
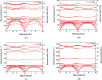
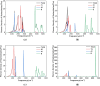
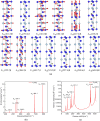
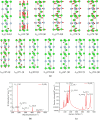
In this paper, the crystal geometry, electronic structure, lattice vibration, Infrared and Raman spectra of ternary layered borides M3AlB2 (M = Ti, Zr, Hf, Ta) are studied by using first principles calculation method based on the density functional theory. The electronic structure of M3AlB2 indicates that they are all electrical conductors, and the d orbitals of Ti, Zr, Hf, and Ta occupy most of the bottom of the conduction band and most of the top of the valence band. Al and B have lower contributions near their Fermi level. The lightweight and stronger chemical bonds of atom B are important factors that correspond to higher levels of peak positions in the Infrared and Raman spectra. However, the vibration frequencies, phonon density of states, and peak positions of Infrared and Raman spectra are significantly lower because of heavier masses and weaker chemical bonds for M and Al atoms. And, there are 6 Infrared active modes A2u and E1u, and 7 Raman active modes, namely A1g, E2g, and E1g corresponding to different vibration frequencies in M3AlB2. Furthermore, the Infrared and Raman spectra of M3AlB2 were obtained respectively, which intuitively provided a reliable Infrared and Raman vibration position and intensity theoretical basis for the experimental study.
Keywords: First-principles; Infrared spectra; MAB phase; Raman spectra.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Barsoum MW, Radovic M. Elastic and mechanical properties of the MAX phases. Annu. Rev. Mater. Res. 2011;41:195–227. doi: 10.1146/annurev-matsci-062910-100448. - DOI
-
- Nie J, Liu S, Zhan X, Ao L, Li L. First-principles study of Hf/Nb/Zr-doped MAX phases Ti3AlC2 and Ti3SiC2. Phys. B. 2019;571:105–111. doi: 10.1016/j.physb.2019.06.052. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

