Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice
- PMID: 38951692
- PMCID: PMC11408255
- DOI: 10.1038/s43587-024-00663-7
Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice
Abstract
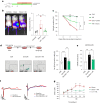
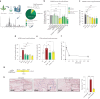
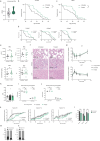
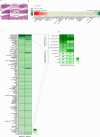
Accumulating senescent cells within tissues contribute to the progression of aging and age-related diseases. Botanical extracts, rich in phytoconstituents, present a useful resource for discovering therapies that could target senescence and thus improve healthspan. Here, we show that daily oral administration of a standardized extract of Salvia haenkei (Haenkenium (HK)) extended lifespan and healthspan of naturally aged mice. HK treatment inhibited age-induced inflammation, fibrosis and senescence markers across several tissues, as well as increased muscle strength and fur thickness compared with age-matched controls. We also found that HK treatment reduced acutely induced senescence by the chemotherapeutic agent doxorubicin, using p16LUC reporter mice. We profiled the constituent components of HK by mass spectrometry, and identified luteolin-the most concentrated flavonoid in HK-as a senomorphic compound. Mechanistically, by performing surface plasmon resonance and in situ proximity ligation assay, we found that luteolin disrupted the p16-CDK6 interaction. This work demonstrates that administration of HK promotes longevity in mice, possibly by modulating cellular senescence and by disrupting the p16-CDK6 interaction.
© 2024. The Author(s).
Conflict of interest statement
A.A. reports consulting fees and grants from IBSA Institute Biochimique SA. A.A. is inventor in patent nos. US9668961B2, EP2762131B1. A.A., A.M.G. and M.M. are inventors in patent nos. US11235016B2, US11235017B2. A.A. and A.M.G. are inventors in patent nos. WO2023280714A1 and WO2023280717A1. A.M.G. is an employee of IBSA Institute Biochimique SA. The remaining authors declare no competing interests.
Figures






References
-
- Lutz, W., Sanderson, W. & Scherbov, S. The coming acceleration of global population ageing. Nature451, 716–719 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

