VAMP2 regulates phase separation of α-synuclein
- PMID: 38951707
- PMCID: PMC11322000
- DOI: 10.1038/s41556-024-01451-6
VAMP2 regulates phase separation of α-synuclein
Abstract
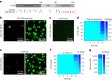
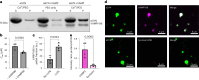
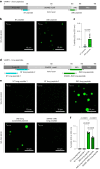
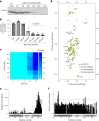
α-Synuclein (αSYN), a pivotal synaptic protein implicated in synucleinopathies such as Parkinson's disease and Lewy body dementia, undergoes protein phase separation. We reveal that vesicle-associated membrane protein 2 (VAMP2) orchestrates αSYN phase separation both in vitro and in cells. Electrostatic interactions, specifically mediated by VAMP2 via its juxtamembrane domain and the αSYN C-terminal region, drive phase separation. Condensate formation is specific for R-SNARE VAMP2 and dependent on αSYN lipid membrane binding. Our results delineate a regulatory mechanism for αSYN phase separation in cells. Furthermore, we show that αSYN condensates sequester vesicles and attract complexin-1 and -2, thus supporting a role in synaptic physiology and pathophysiology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys.11, 899–904 (2015). 10.1038/nphys3532 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- DHF/R1/201228/Royal Society
- RPG-2022-257/Leverhulme Trust
- 900325/CUH | Addenbrooke's Charitable Trust, Cambridge University Hospitals (Addenbrooke's Charitable Trust, Cambridge University Hospitals NHS Foundation Trust)
- Award 900325/CUH | Addenbrooke's Charitable Trust, Cambridge University Hospitals (Addenbrooke's Charitable Trust, Cambridge University Hospitals NHS Foundation Trust)
- MR/T012412/1/RCUK | Medical Research Council (MRC)
LinkOut - more resources
Full Text Sources
Miscellaneous

