Additively manufactured high-entropy alloys for hydrogen storage: Predictions
- PMID: 38952385
- PMCID: PMC11215296
- DOI: 10.1016/j.heliyon.2024.e32715
Additively manufactured high-entropy alloys for hydrogen storage: Predictions
Abstract
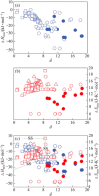
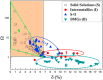
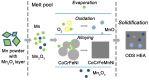
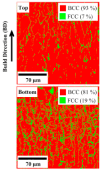
This review paper covers an analysis of the empirical calculations, additive manufacturing (AM) and hydrogen storage of refractory high-entropy alloys undertaken to determine the structural compositions, particularly focusing on their applicability in research and experimental settings. The inventors of multi-component high-entropy alloys (HEAs) calculated that trillions of materials could be manufactured from elements in the periodic table, estimating a vast number, N = 10^100, using Stirling's approximation. The significant contribution of semi-empirical parameters such as Gibbs free energy ΔG, enthalpy of mixing ΔH mix , entropy of mixing ΔS mix , atomic size difference Δδ, valence electron concentration VEC, and electronegativity difference Δχ are to predict BCC and/or FCC phases in HEAs. Additive manufacturing facilitates the determination of refractory HEAs systems with the most stable solid-solution and single-phase, and their subsequent hydrogen storage capabilities. Hydride materials, especially those from HEAs manufactured by AM as bulk and solid materials, have great potential for H2 storage, with storage capacities that can be as high as 1.81 wt% of H2 adsorbed for a ZrTiVCrFeNi system. Furthermore, laser metal deposition (LMD) is the most commonly employed technique for fabricating refractory high entropy alloys, surpassing other methods in usage, thus making it particularly suitable for H2 storage.
Keywords: Additive manufacturing; Empirical models; High-entropy alloys; Hydrogen storage; Phase prediction; Refractory elements.
© 2024 The Author. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Cantor B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021;120
-
- Kunce I., Polanski M., Bystrzycki J. Structure and hydrogen storage properties of a high entropy ZrTiVCrFeNi alloy synthesized using Laser Engineered Net Shaping (LENS) Int. J. Hydrogen Energy. 2013;38:12180–12189.
-
- Yeh J.W., Chen S.K., Lin S.J., Gan J.Y., Chin T.S., Shun T.T., Tsau C.H., Chang S.Y. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004;6:299–303.
-
- Cantor B., Chang I.T.H., Knight P., Vincent A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004;375–377:213–218.
-
- Yang F., Wang J., Zhang Y., Wu Z., Zhang Z., Zhao F., Huot J., Grobivć Novaković J., Novaković N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: a review. Int. J. Hydrogen Energy. 2022;47:11236–11249.
Publication types
LinkOut - more resources
Full Text Sources

