Combined aqupla, paclitaxel liposome, and docetaxel treatment: survival and biomarker outcomes in recurrent ovarian cancer patients
- PMID: 38952549
- PMCID: PMC11215079
- DOI: 10.3389/fonc.2024.1422117
Combined aqupla, paclitaxel liposome, and docetaxel treatment: survival and biomarker outcomes in recurrent ovarian cancer patients
Abstract
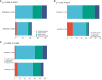
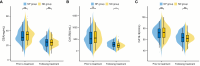
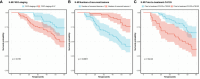
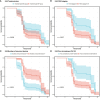
As one lethal malignancy in women's reproductive systems, ovarian cancer (OC) is frequently detected at an advanced phase during diagnosis. when the disease has spread widely. The absence of obvious symptoms and powerful screening tools in the early stages makes treatment difficult and the prognosis poor. Despite the clinical remission that can be achieved in some patients after initial treatment, the recurrence rate is conspicuous, posing a considerable challenge in treating recurrent OC (ROC). In the retrospective analysis, we compared the effects of two treatment regimens, aqupla combined with paclitaxel liposome (NP group) versus aqupla combined with docetaxel (ND group), on survival and biomarkers in patients with ROC. The study included 121 OC patients, and clinical data were collected through an electronic medical record system, outpatient review records, and a follow-up record system. The results revealed a notably higher overall remission rate in the ND group than the NP group, but revealed no notable inter-group discrepancy in toxicities, implying that the aqupla combined with docetaxel regimen may be more effective in platinum-sensitive ROC patients. Additionally, post-treatment CA125 levels were lower in patients in the ND group, suggesting that the regimen may be more effective in reducing tumour load. Survival analysis further revealed that treatment regimen, FIGO stage, number of recurrent lesions, and pretreatment CA125 level were independent prognostic factors affecting patients' 5-year OS and PFS. Overall for ROC patients, especially platinum-sensitive patients, the aqupla in combination with docetaxel regimen provided an improved survival benefit with a comparable safety profile, highlighting the importance of individualised treatment strategies.
Keywords: aqupla; biomarkers; docetaxel; paclitaxel liposome; recurrent ovarian cancer; survival.
Copyright © 2024 Yang, Zhang, Zhang, Zhu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

