Altered correlation of concurrently recorded EEG-fMRI connectomes in temporal lobe epilepsy
- PMID: 38952816
- PMCID: PMC11142634
- DOI: 10.1162/netn_a_00362
Altered correlation of concurrently recorded EEG-fMRI connectomes in temporal lobe epilepsy
Abstract
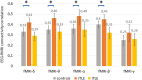
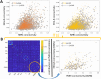
Whole-brain functional connectivity networks (connectomes) have been characterized at different scales in humans using EEG and fMRI. Multimodal epileptic networks have also been investigated, but the relationship between EEG and fMRI defined networks on a whole-brain scale is unclear. A unified multimodal connectome description, mapping healthy and pathological networks would close this knowledge gap. Here, we characterize the spatial correlation between the EEG and fMRI connectomes in right and left temporal lobe epilepsy (rTLE/lTLE). From two centers, we acquired resting-state concurrent EEG-fMRI of 35 healthy controls and 34 TLE patients. EEG-fMRI data was projected into the Desikan brain atlas, and functional connectomes from both modalities were correlated. EEG and fMRI connectomes were moderately correlated. This correlation was increased in rTLE when compared to controls for EEG-delta/theta/alpha/beta. Conversely, multimodal correlation in lTLE was decreased in respect to controls for EEG-beta. While the alteration was global in rTLE, in lTLE it was locally linked to the default mode network. The increased multimodal correlation in rTLE and decreased correlation in lTLE suggests a modality-specific lateralized differential reorganization in TLE, which needs to be considered when comparing results from different modalities. Each modality provides distinct information, highlighting the benefit of multimodal assessment in epilepsy.
Keywords: Concurrent EEG-fMRI; Functional connectome; Multimodal data integration; Resting state; Temporal lobe epilepsy.
Plain language summary
The relationship between resting-state hemodynamic (fMRI) and electrophysiological (EEG) connectivity has been investigated in healthy subjects, but this relationship is unknown in patients with left and right temporal lobe epilepsies (l/rTLE). Does the magnitude of the relationship differ between healthy subjects and patients? What role does the laterality of the epileptic focus play? What are the spatial contributions to this relationship? Here we use concurrent EEG-fMRI recordings of 65 subjects from two centers (35 controls, 34 TLE patients), to assess the correlation between EEG and fMRI connectivity. For all datasets, frequency-specific changes in cross-modal correlation were seen in lTLE and rTLE. EEG and fMRI connectivities do not measure perfectly overlapping brain networks and provide distinct information on brain networks altered in TLE, highlighting the benefit of multimodal assessment to inform about normal and pathological brain function.
© 2024 Massachusetts Institute of Technology.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures

References
-
- Baillet, S., Mosher, J. C., & Leahy, R. M. (2001). Electromagnetic brain mapping. IEEE Signal Processing Magazine, 18(6), 14–30. 10.1109/79.962275 - DOI
-
- Bartolomei, F., Guye, M., & Wendling, F. (2013). Abnormal binding and disruption in large scale networks involved in human partial seizures. EPJ Nonlinear Biomedical Physics, 1(1), 1–16. 10.1140/epjnbp11 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
