Effect of finerenone on nephrotic syndrome in patients with diabetic kidney disease
- PMID: 38952893
- PMCID: PMC11215105
- DOI: 10.1016/j.metop.2024.100294
Effect of finerenone on nephrotic syndrome in patients with diabetic kidney disease
Abstract
Introduction: Diabetic kidney disease (DKD) is an important complication of diabetes as it results in end-stage renal disease; hence, several drugs have been developed for its treatment. However, even with treatment with renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors, the residual risk of DKD remains. While this risk is an issue, the renoprotective effects of finerenone, a novel non-steroidal mineralocorticoid receptor antagonist, are becoming evident. High proteinuria increases the risk of cardiovascular death as well as renal failure. Hence, it is especially important to address cases of urine protein to nephrotic levels in DKD, however, no previous studies have assessed the safety and efficacy of finerenone in patients with DKD and nephrotic syndrome. Therefore, this study aimed to assess whether finerenone has a renoprotective effect in advanced DKD complicated by nephrotic syndrome.
Methods: Nine patients with DKD and nephrotic syndrome who received 10-20 mg/day of finerenone were retrospectively analyzed. The average observation period was 9.7 ± 3.4 months. Patients with serum potassium levels greater than 5.0 mEq/L at the start of finelenone were excluded. Changes in urinary protein levels, estimated glomerular filtration rate (eGFR), and serum potassium levels were studied before and after finerenone administration.
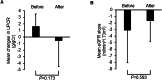
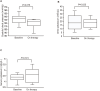
Results: The mean changes in the urinary protein creatinine ratio (UPCR) at baseline were 6.6 ± 2.0. After finerenone treatment, the mean UPCR decreased to -0.6 ± 3.9; however, this change was not statistically significant.The eGFR decline slope also tended to decrease with finerenone treatment (before vs. after: 3.1 ± 4.9 vs. -1.7 ± 3.2 mL/min/1.73 m2. Furthermore, finerenone did not increase serum potassium levels.
Conclusions: Patients treated with finerenone showed decreased UPCR; hence, it is suggested that finerenone may be effective in treating nephrotic syndrome in patients with DKD. These findings may be applicable to real-world clinical settings. Nonetheless, it is important to note that this study was a retrospective analysis of a single-center cohort and had a limited sample size, highlighting the need for additional large-scale investigations.
Keywords: Diabetic kidney disease; Estimated glomerular filtration rate; Finerenone; Nephrotic syndrome; UPCR.
© 2024 The Authors.
Conflict of interest statement
A. Mima received speaker honorariums from Novartis, Kyowa Kirin, Bayer, Eli Lilly, Mochida, and Boehringer Ingelheim. A. Mima has also received research grants from Sumitomo Pharma, Chugai, Torii, and Mochida.
Figures
Similar articles
-
Effectiveness and safety of finerenone in diabetic kidney disease patients: a real-world observational study from China.Ren Fail. 2024 Dec;46(2):2400541. doi: 10.1080/0886022X.2024.2400541. Epub 2024 Sep 9. Ren Fail. 2024. PMID: 39248389 Free PMC article.
-
Investigating the use of finerenone in children with chronic kidney disease and proteinuria: design of the FIONA and open-label extension studies.Trials. 2024 Mar 21;25(1):203. doi: 10.1186/s13063-024-08021-z. Trials. 2024. PMID: 38509517 Free PMC article. Clinical Trial.
-
Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO-DKD compared to reported CREDENCE results.Nephrol Dial Transplant. 2022 Jun 23;37(7):1261-1269. doi: 10.1093/ndt/gfab336. Nephrol Dial Transplant. 2022. PMID: 34850173 Free PMC article. Clinical Trial.
-
Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease.Endocrinol Metab (Seoul). 2023 Feb;38(1):43-55. doi: 10.3803/EnM.2022.1629. Epub 2023 Feb 27. Endocrinol Metab (Seoul). 2023. PMID: 36891650 Free PMC article. Review.
-
Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease.Postgrad Med. 2023 Apr;135(3):224-233. doi: 10.1080/00325481.2022.2060598. Epub 2022 Apr 20. Postgrad Med. 2023. PMID: 35392754 Review.
Cited by
-
Early Clinical Experience of Finerenone in People with Chronic Kidney Disease and Type 2 Diabetes in Japan-A Multi-Cohort Study from the FOUNTAIN (FinerenOne mUltidatabase NeTwork for Evidence generAtIoN) Platform.J Clin Med. 2024 Aug 28;13(17):5107. doi: 10.3390/jcm13175107. J Clin Med. 2024. PMID: 39274317 Free PMC article.
-
Potential Role of Mineralocorticoid Receptor Antagonists in Nondiabetic Chronic Kidney Disease and Glomerular Disease.Clin J Am Soc Nephrol. 2024 Nov 1;19(11):1499-1512. doi: 10.2215/CJN.0000000000000540. Epub 2024 Jul 22. Clin J Am Soc Nephrol. 2024. PMID: 39037799 Review.
-
Effectiveness of finerenone in Chinese patients with type 2 diabetes mellitus and chronic kidney disease with microalbuminuria: A retrospective real-world study.J Diabetes Investig. 2025 Jun;16(6):1028-1033. doi: 10.1111/jdi.70023. Epub 2025 Mar 15. J Diabetes Investig. 2025. PMID: 40087914 Free PMC article.
-
Efficacy and safety of finerenone in non-diabetic CKD patients: a single-center, real-world, retrospective study.BMC Nephrol. 2025 Jul 1;26(1):323. doi: 10.1186/s12882-025-04241-w. BMC Nephrol. 2025. PMID: 40597854 Free PMC article.
References
-
- Diabetes C., Complications Trial Research G., Nathan D.M., Genuth S., Lachin J., Cleary P., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. - PubMed
-
- Bailey C.J., Grant P.J. The UK prospective diabetes study. Lancet. 1998;352:1932. author reply 4. - PubMed
-
- Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. - PubMed
-
- Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B., et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. - PubMed
-
- Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

