Impacts of polyethylene glycol (PEG) dispersity on protein adsorption, pharmacokinetics, and biodistribution of PEGylated gold nanoparticles
- PMID: 38952930
- PMCID: PMC11216039
- DOI: 10.1039/d4ra03153a
Impacts of polyethylene glycol (PEG) dispersity on protein adsorption, pharmacokinetics, and biodistribution of PEGylated gold nanoparticles
Abstract
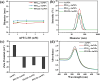
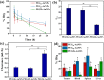
PEGylated gold nanoparticles (PEG-AuNPs) are widely used in drug delivery, imaging and diagnostics, therapeutics, and biosensing. However, the effect of PEG dispersity on the molecular weight (M W) distribution of PEG grafted onto AuNP surfaces has been rarely reported. This study investigates the effect of PEG dispersity on the M W distribution of PEG grafted onto AuNP surfaces and its subsequent impact on protein adsorption and pharmacokinetics, by modifying AuNPs with monodisperse PEG methyl ether thiols (mPEG n -HS, n = 36, 45) and traditional polydisperse mPEG2k-SH (M W = 1900). Polydisperse PEG-AuNPs favor the enrichment of lower M W PEG fractions on their surface due to the steric hindrance effect, which leads to increased protein adsorption. In contrast, monodisperse PEG-AuNPs have a uniform length of PEG outlayer, exhibiting markedly lower yet constant protein adsorption. Pharmacokinetics analysis in tumor-bearing mice demonstrated that monodisperse PEG-AuNPs possess a significantly prolonged blood circulation half-life and enhanced tumor accumulation compared with their polydisperse counterpart. These findings underscore the critical, yet often underestimated, impacts of PEG dispersity on the in vitro and in vivo behavior of PEG-AuNPs, highlighting the role of monodisperse PEG in enhancing therapeutic nanoparticle performance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Chen Y. Feng X. Int. J. Pharm. 2022;625:122122. - PubMed
-
- Urso A. Meloni F. Malatesta M. Latorre R. Damoci C. Crapanzano J. Pandolfi L. Giustra M. D. Pearson M. Colombo M. Schilling K. Glabonjat R. A. D'Ovidio F. Nanomedicine. 2023;18:317–330. - PubMed
-
- Yao T. Wang R. Meng Y. Hun X. ACS Appl. Mater. Interfaces. 2021;13:26515–26521. - PubMed
LinkOut - more resources
Full Text Sources

