Effect of glucocorticoid blockade on inflammatory responses to acute sleep fragmentation in male mice
- PMID: 38952964
- PMCID: PMC11216221
- DOI: 10.7717/peerj.17539
Effect of glucocorticoid blockade on inflammatory responses to acute sleep fragmentation in male mice
Abstract
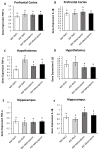
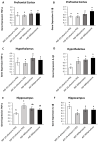
The association between sleep and the immune-endocrine system is well recognized, but the nature of that relationship is not well understood. Sleep fragmentation induces a pro-inflammatory response in peripheral tissues and brain, but it also activates the hypothalamic-pituitary-adrenal (HPA) axis, releasing glucocorticoids (GCs) (cortisol in humans and corticosterone in mice). It is unclear whether this rapid release of glucocorticoids acts to potentiate or dampen the inflammatory response in the short term. The purpose of this study was to determine whether blocking or suppressing glucocorticoid activity will affect the inflammatory response from acute sleep fragmentation (ASF). Male C57BL/6J mice were injected i.p. with either 0.9% NaCl (vehicle 1), metyrapone (a glucocorticoid synthesis inhibitor, dissolved in vehicle 1), 2% ethanol in polyethylene glycol (vehicle 2), or mifepristone (a glucocorticoid receptor antagonist, dissolved in vehicle 2) 10 min before the start of ASF or no sleep fragmentation (NSF). After 24 h, samples were collected from brain (prefrontal cortex, hypothalamus, hippocampus) and periphery (liver, spleen, heart, and epididymal white adipose tissue (EWAT)). Proinflammatory gene expression (TNF-α and IL-1β) was measured, followed by gene expression analysis. Metyrapone treatment affected pro-inflammatory cytokine gene expression during ASF in some peripheral tissues, but not in the brain. More specifically, metyrapone treatment suppressed IL-1β expression in EWAT during ASF, which implies a pro-inflammatory effect of GCs. However, in cardiac tissue, metyrapone treatment increased TNF-α expression in ASF mice, suggesting an anti-inflammatory effect of GCs. Mifepristone treatment yielded more significant results than metyrapone, reducing TNF-α expression in liver (only NSF mice) and cardiac tissue during ASF, indicating a pro-inflammatory role. Conversely, in the spleen of ASF-mice, mifepristone increased pro-inflammatory cytokines (TNF-α and IL-1β), demonstrating an anti-inflammatory role. Furthermore, irrespective of sleep fragmentation, mifepristone increased pro-inflammatory cytokine gene expression in heart (IL-1β), pre-frontal cortex (IL-1β), and hypothalamus (IL-1β). The results provide mixed evidence for pro- and anti-inflammatory functions of corticosterone to regulate inflammatory responses to acute sleep loss.
Keywords: Acute Sleep Fragmentation; Glucocorticoids; Metyrapone; Mifepristone; Pro-inflammatory cytokines.
©2024 Hasan et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Ajibare AJ, Ayodele OD, Olayaki LA. Mifepristone ameliorates sleep deprivation - induced oxidative stress in the testis of rats. African Journal of Biomedical Research. 2020;23(2):239–245.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

