Different localization of P2X4 and P2X7 receptors in native mouse lung - lack of evidence for a direct P2X4-P2X7 receptor interaction
- PMID: 38953020
- PMCID: PMC11215518
- DOI: 10.3389/fimmu.2024.1425938
Different localization of P2X4 and P2X7 receptors in native mouse lung - lack of evidence for a direct P2X4-P2X7 receptor interaction
Abstract
Introduction: P2X receptors are a family of homo- and heterotrimeric cation channels gated by extracellular ATP. The P2X4 and P2X7 subunits show overlapping expression patterns and have been involved in similar physiological processes, such as pain and inflammation as well as various immune cell functions. While formation of P2X2/P2X3 heterotrimers produces a distinct pharmacological phenotype and has been well established, functional identification of a P2X4/P2X7 heteromer has been difficult and evidence for and against a physical association has been found. Most of this evidence stems, however, from in vitro model systems.
Methods: Here, we used a P2X7-EGFP BAC transgenic mouse model as well as P2X4 and P2X7 knock-out mice to re-investigate a P2X4-P2X7 interaction in mouse lung by biochemical and immunohistochemical experiments as well as quantitative expression analysis.
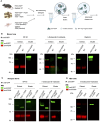
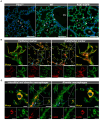
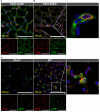
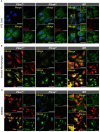
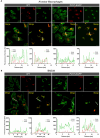
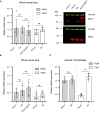
Results: No detectable amounts of P2X4 could be co-purified from mouse lung via P2X7-EGFP. In agreement with these findings, immuno-histochemical analysis using a P2X7-specific nanobody revealed only limited overlap in the cellular and subcellular localizations of P2X4 and P2X7 in both the native lung tissue and primary cells. Comparison of P2X4 and P2X7 transcript and protein levels in the respective gene-deficient and wild type mice showed no mutual interrelation between their expression levels in whole lungs. However, a significantly reduced P2rx7 expression was found in alveolar macrophages of P2rx4 -/- mice.
Discussion: In summary, our detailed analysis of the cellular and subcellular P2X4 and P2X7 localization and expression does not support a physiologically relevant direct association of P2X4 and P2X7 subunits or receptors in vivo.
Keywords: BAC transgenic P2X7-EGFP mouse; P2X4 receptor; P2X7 receptor; functional interaction; heteromerization; lung epithelial cells; macrophage; nanobody.
Copyright © 2024 Sierra-Marquez, Schaller, Sassenbach, Ramírez-Fernández, Alt, Rissiek, Zimmer, Schredelseker, Hector, Stähler, Koch-Nolte, Staab-Weijnitz, Dietrich, Kopp and Nicke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

