Ileal Paneth Cell Phenotype is a Cellular Biomarker for Pouch Complications in Ulcerative Colitis
- PMID: 38953127
- PMCID: PMC11637519
- DOI: 10.1093/ecco-jcc/jjae105
Ileal Paneth Cell Phenotype is a Cellular Biomarker for Pouch Complications in Ulcerative Colitis
Abstract
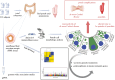
Background & aims: Biomarkers that integrate genetic and environmental factors and predict outcome in complex immune diseases such as inflammatory bowel disease (IBD; including Crohn's disease [CD] and ulcerative colitis [UC]) are needed. We showed that morphologic patterns of ileal Paneth cells (Paneth cell phenotype [PCP]; a surrogate for PC function) is one such cellular biomarker for CD. Given the shared features between CD and UC, we hypothesized that PCP is also associated with molecular/genetic features and outcome in UC. Because PC density is highest in the ileum, we further hypothesized that PCP predicts outcome in UC subjects who underwent total colectomy and ileal pouch-anal anastomosis (IPAA).
Methods: Uninflamed ileal resection margins from UC subjects with colectomy and IPAA were used for PCP and transcriptomic analyses. PCP was defined using defensin 5 immunofluorescence. Genotyping was performed using Immunochip. UC transcriptomic and genotype associations of PCP were incorporated with data from CD subjects to identify common IBD-related pathways and genes that regulate PCP.
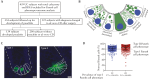
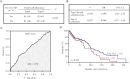
Results: The prevalence of abnormal ileal PCP was 27%, comparable to that seen in CD. Combined analysis of UC and CD subjects showed that abnormal PCP was associated with transcriptomic pathways of secretory granule maturation and polymorphisms in innate immunity genes. Abnormal ileal PCP at the time of colectomy was also associated with pouch complications including de novo CD in the pouch and time to first episode of pouchitis.
Conclusions: Ileal PCP is biologically and clinically relevant in UC and can be used as a biomarker in IBD.
Keywords: de novo Crohn’s disease; ileal pouch-anal anastomosis; pouchitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
TCL has research contracts from Interline Therapeutics and Denali Therapeutics. DPBM owns stock in Prometheus Biosciences. DPBM has consulted for Gilead, Pfizer, Boehringer Ingelheim, Qu Biologics, Bridge Biotherapeutics, Takeda, Palatin Technologies, and received grant support from Janssen. All other authors have no conflict of interest to report.
Figures


References
-
- Mosli MH, Sandborn WJ, Kim RB, Khanna R, Al-Judaibi B, Feagan BG.. Toward a personalized medicine approach to the management of inflammatory bowel disease. Am J Gastroenterol 2014;109:994–1004. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

