Directed evolution of hyperactive integrases for site specific insertion of transgenes
- PMID: 38953167
- PMCID: PMC11317131
- DOI: 10.1093/nar/gkae534
Directed evolution of hyperactive integrases for site specific insertion of transgenes
Abstract
The ability to deliver large transgenes to a single genomic sequence with high efficiency would accelerate biomedical interventions. Current methods suffer from low insertion efficiency and most rely on undesired double-strand DNA breaks. Serine integrases catalyze the insertion of large DNA cargos at attachment (att) sites. By targeting att sites to the genome using technologies such as prime editing, integrases can target safe loci while avoiding double-strand breaks. We developed a method of phage-assisted continuous evolution we call IntePACE, that we used to rapidly perform hundreds of rounds of mutagenesis to systematically improve activity of PhiC31 and Bxb1 serine integrases. Novel hyperactive mutants were generated by combining synergistic mutations resulting in integration of a multi-gene cargo at rates as high as 80% of target chromosomes. Hyperactive integrases inserted a 15.7 kb therapeutic DNA cargo containing von Willebrand Factor. This technology could accelerate gene delivery therapeutics and our directed evolution strategy can easily be adapted to improve novel integrases from nature.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
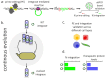

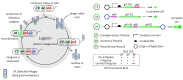
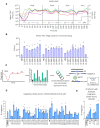
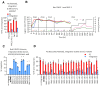
Update of
-
Directed evolution of hyperactive integrases for site specific insertion of transgenes.bioRxiv [Preprint]. 2024 Jun 11:2024.06.10.598370. doi: 10.1101/2024.06.10.598370. bioRxiv. 2024. Update in: Nucleic Acids Res. 2024 Aug 12;52(14):e64. doi: 10.1093/nar/gkae534. PMID: 38915697 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

