Rare Variant in MRC2 Associated With Familial Supraventricular Tachycardia and Wolff-Parkinson-White Syndrome
- PMID: 38953222
- PMCID: PMC11335451
- DOI: 10.1161/CIRCGEN.124.004614
Rare Variant in MRC2 Associated With Familial Supraventricular Tachycardia and Wolff-Parkinson-White Syndrome
Abstract
Background: Accessory pathways are a common cause of supraventricular tachycardia (SVT) and can lead to sudden cardiac death in otherwise healthy children and adults when associated with Wolff-Parkinson-White syndrome. The goal of this study was to identify genetic variants within a large family with structurally normal hearts affected by SVT and Wolff-Parkinson-White syndrome and determine causality of the gene deficit in a corresponding mouse model.
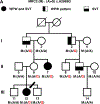
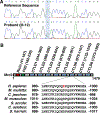
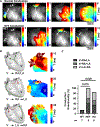
Methods: Whole exome sequencing performed on 2 distant members of a 3-generation family in which multiple members were affected by SVT or Wolff-Parkinson-White pattern (preexcitation) on ECG identified MRC2 as a candidate gene. Serial electrocardiograms, intracardiac electrophysiology studies, echocardiography, optical mapping studies, and histology were performed on both Mrc2 mutant and WT (wild-type) mice.
Results: A rare HET (heterozygous) missense variant c.2969A>G;p.Glu990Gly (E990G) in MRC2 was identified as the leading candidate gene variant segregating with the cardiac phenotype following an autosomal-dominant Mendelian trait segregation pattern with variable expressivity. In vivo electrophysiology studies revealed reentrant SVT in E990G mice. Optical mapping studies in E990G mice demonstrated abnormal retrograde conduction, suggesting the presence of an accessory pathway. Histological analysis of E990G mouse hearts showed a disordered ECM (extracellular matrix) in the annulus fibrosus. Finally, Mrc2 knockdown in human cardiac fibroblasts enhanced accelerated cell migration.
Conclusions: This study identified a rare nonsynonymous variant in the MRC2 gene in individuals with familial reentrant SVT, Wolff-Parkinson-White ECG pattern, and structurally normal hearts. Furthermore, Mrc2 knock-in mice revealed an increased incidence of reentrant SVT and bypass tract formation in the setting of preserved cardiac structure and function.
Keywords: Wolff-Parkinson-White syndrome; cell movement; fibroblasts; fibrosis; tachycardia, supraventricular.
Conflict of interest statement
Dr Lupski has stock ownership in 23andMe (Sunnyvale, CA), is a paid consultant for the Regeneron Genetics Center (Tarrytown, NY), and is a coinventor on multiple US and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics (BG) Laboratories (Houston, TX). Dr Lupski serves on the Scientific Advisory Board of BG. Dr Wehrens is a cofounder and board member of Elex Biotech LLC, a start-up company developing RyR2 (ryanodine receptor type-2) modifying drugs for heart disease. Dr Wehrens is also a consultant at Pfizer and Rocket Pharmaceuticals. The other authors report no conflicts.
Figures





References
-
- Coban-Akdemir ZH, Charng WL, Azamian M, Paine IS, Punetha J, Grochowski CM, Gambin T, Valdes SO, Cannon B, Zapata G, et al. Wolff-Parkinson-White syndrome: De novo variants and evidence for mutational burden in genes associated with atrial fibrillation. Am J Med Genet A. 2020;182:1387–1399. - PMC - PubMed
-
- Etheridge SP, Escudero CA, Blaufox AD, Law IH, Dechert-Crooks BE, Stephenson EA, Dubin AM, Ceresnak SR, Motonaga KS, Skinner JR, et al. Life-Threatening Event Risk in Children With Wolff-Parkinson-White Syndrome: A Multicenter International Study. JACC Clin Electrophysiol. 2018;4:433–444. - PubMed
-
- Kolditz DP, Wijffels MC, Blom NA, van der Laarse A, Markwald RR, Schalij MJ, Gittenberger-de Groot AC. Persistence of functional atrioventricular accessory pathways in postseptated embryonic avian hearts: implications for morphogenesis and functional maturation of the cardiac conduction system. Circulation. 2007;115:17–26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

