Metabolic stress induces a double-positive feedback loop between AMPK and SQSTM1/p62 conferring dual activation of AMPK and NFE2L2/NRF2 to synergize antioxidant defense
- PMID: 38953310
- PMCID: PMC11572134
- DOI: 10.1080/15548627.2024.2374692
Metabolic stress induces a double-positive feedback loop between AMPK and SQSTM1/p62 conferring dual activation of AMPK and NFE2L2/NRF2 to synergize antioxidant defense
Abstract
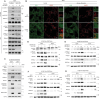
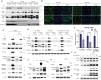
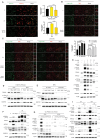
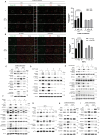
Co-occurring mutations in KEAP1 in STK11/LKB1-mutant NSCLC activate NFE2L2/NRF2 to compensate for the loss of STK11-AMPK activity during metabolic adaptation. Characterizing the regulatory crosstalk between the STK11-AMPK and KEAP1-NFE2L2 pathways during metabolic stress is crucial for understanding the implications of co-occurring mutations. Here, we found that metabolic stress increased the expression and phosphorylation of SQSTM1/p62, which is essential for the activation of NFE2L2 and AMPK, synergizing antioxidant defense and tumor growth. The SQSTM1-driven dual activation of NFE2L2 and AMPK was achieved by inducing macroautophagic/autophagic degradation of KEAP1 and facilitating the AXIN-STK11-AMPK complex formation on the lysosomal membrane, respectively. In contrast, the STK11-AMPK activity was also required for metabolic stress-induced expression and phosphorylation of SQSTM1, suggesting a double-positive feedback loop between AMPK and SQSTM1. Mechanistically, SQSTM1 expression was increased by the PPP2/PP2A-dependent dephosphorylation of TFEB and TFE3, which was induced by the lysosomal deacidification caused by low glucose metabolism and AMPK-dependent proton reduction. Furthermore, SQSTM1 phosphorylation was increased by MAP3K7/TAK1, which was activated by ROS and pH-dependent secretion of lysosomal Ca2+. Importantly, phosphorylation of SQSTM1 at S24 and S226 was critical for the activation of AMPK and NFE2L2. Notably, the effects caused by metabolic stress were abrogated by the protons provided by lactic acid. Collectively, our data reveal a novel double-positive feedback loop between AMPK and SQSTM1 leading to the dual activation of AMPK and NFE2L2, potentially explaining why co-occurring mutations in STK11 and KEAP1 happen and providing promising therapeutic strategies for lung cancer.Abbreviations: AMPK: AMP-activated protein kinase; BAF1: bafilomycin A1; ConA: concanamycin A; DOX: doxycycline; IP: immunoprecipitation; KEAP1: kelch like ECH associated protein 1; LN: low nutrient; MAP3K7/TAK1: mitogen-activated protein kinase kinase kinase 7; MCOLN1/TRPML1: mucolipin TRP cation channel 1; MEFs: mouse embryonic fibroblasts; MTORC1: mechanistic target of rapamycin kinase complex 1; NAC: N-acetylcysteine; NFE2L2/NRF2: NFE2 like bZIP transcription factor 2; NSCLC: non-small cell lung cancer; PRKAA/AMPKα: protein kinase AMP-activated catalytic subunit alpha; PPP2/PP2A: protein phosphatase 2; ROS: reactive oxygen species; PPP3/calcineurin: protein phosphatase 3; RPS6KB1/p70S6K: ribosomal protein S6 kinase B1; SQSTM1/p62: sequestosome 1; STK11/LKB1: serine/threonine kinase 11; TCL: total cell lysate; TFEB: transcription factor EB; TFE3: transcription factor binding to IGHM enhancer 3; V-ATPase: vacuolar-type H+-translocating ATPase.
Keywords: AXIN; KEAP1; STK11/LKB1; lysosomal stress; metabolic stress; oxidative stress.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
USP13 facilitates a ferroptosis-to-autophagy switch by activation of the NFE2L2/NRF2-SQSTM1/p62-KEAP1 axis dependent on the KRAS signaling pathway.Autophagy. 2025 Mar;21(3):565-582. doi: 10.1080/15548627.2024.2410619. Epub 2024 Oct 10. Autophagy. 2025. PMID: 39360581
-
Serine ubiquitination of SQSTM1 regulates NFE2L2-dependent redox homeostasis.Autophagy. 2025 Feb;21(2):407-423. doi: 10.1080/15548627.2024.2404375. Epub 2024 Sep 27. Autophagy. 2025. PMID: 39291751 Free PMC article.
-
TRIM21-mediated ubiquitination of SQSTM1/p62 abolishes its Ser403 phosphorylation and enhances palmitic acid cytotoxicity.Autophagy. 2025 Jan;21(1):178-190. doi: 10.1080/15548627.2024.2394308. Epub 2024 Sep 10. Autophagy. 2025. PMID: 39172027 Free PMC article.
-
Genetic drivers of tumor microenvironment and immunotherapy resistance in non-small cell lung cancer: the role of KEAP1, SMARCA4, and PTEN mutations.J Immunother Cancer. 2025 Aug 5;13(8):e012288. doi: 10.1136/jitc-2025-012288. J Immunother Cancer. 2025. PMID: 40764107 Free PMC article. Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
Cited by
-
New Insights into AMPK, as a Potential Therapeutic Target in Metabolic Dysfunction-Associated Steatotic Liver Disease and Hepatic Fibrosis.Biomol Ther (Seoul). 2025 Jan 1;33(1):18-38. doi: 10.4062/biomolther.2024.188. Epub 2024 Dec 20. Biomol Ther (Seoul). 2025. PMID: 39702310 Free PMC article. Review.
-
Unveiling ferroptosis: a new frontier in skin disease research.Front Immunol. 2024 Oct 4;15:1485523. doi: 10.3389/fimmu.2024.1485523. eCollection 2024. Front Immunol. 2024. PMID: 39430757 Free PMC article. Review.
-
A CRISPR-edited isoform of the AMPK kinase LKB1 improves the response to cisplatin in A549 lung cancer cells.J Biol Chem. 2025 Mar;301(3):108308. doi: 10.1016/j.jbc.2025.108308. Epub 2025 Feb 13. J Biol Chem. 2025. PMID: 39955067 Free PMC article.
-
Divergent Processing of Cell Stress Signals as the Basis of Cancer Progression: Licensing NFκB on Chromatin.Int J Mol Sci. 2024 Aug 7;25(16):8621. doi: 10.3390/ijms25168621. Int J Mol Sci. 2024. PMID: 39201306 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
