Host cells reprogram lipid droplet synthesis through YY1 to resist PRRSV infection
- PMID: 38953350
- PMCID: PMC11323570
- DOI: 10.1128/mbio.01549-24
Host cells reprogram lipid droplet synthesis through YY1 to resist PRRSV infection
Abstract
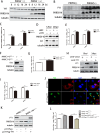
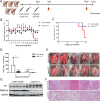
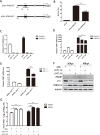
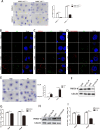
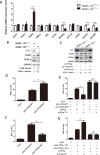
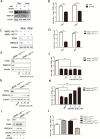
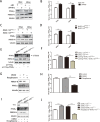
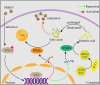
Metabolism in host cells can be modulated after viral infection, favoring viral survival or clearance. Here, we report that lipid droplet (LD) synthesis in host cells can be modulated by yin yang 1 (YY1) after porcine reproductive and respiratory syndrome virus (PRRSV) infection, resulting in active antiviral activity. As a ubiquitously distributed transcription factor, there was increased expression of YY1 upon PRRSV infection both in vitro and in vivo. YY1 silencing promoted the replication of PRRSV, whereas YY1 overexpression inhibited PRRSV replication. PRRSV infection led to a marked increase in LDs, while YY1 knockout inhibited LD synthesis, and YY1 overexpression enhanced LD accumulation, indicating that YY1 reprograms PRRSV infection-induced intracellular LD synthesis. We also showed that the viral components do not colocalize with LDs during PRRSV infection, and the effect of exogenously induced LD synthesis on PRRSV replication is nearly lethal. Moreover, we demonstrated that YY1 affects the synthesis of LDs by regulating the expression of lipid metabolism genes. YY1 negatively regulates the expression of fatty acid synthase (FASN) to weaken the fatty acid synthesis pathway and positively regulates the expression of peroxisome proliferator-activated receptor gamma (PPARγ) to promote the synthesis of LDs, thus inhibiting PRRSV replication. These novel findings indicate that YY1 plays a crucial role in regulating PRRSV replication by reprogramming LD synthesis. Therefore, our study provides a novel mechanism of host resistance to PRRSV and suggests potential new antiviral strategies against PRRSV infection.IMPORTANCEPorcine reproductive and respiratory virus (PRRSV) has caused incalculable economic damage to the global pig industry since it was first discovered in the 1980s. However, conventional vaccines do not provide satisfactory protection. It is well known that viruses are parasitic pathogens, and the completion of their replication life cycle is highly dependent on host cells. A better understanding of host resistance to PRRSV infection is essential for developing safe and effective strategies to control PRRSV. Here, we report a crucial host antiviral molecule, yin yang 1 (YY1), which is induced to be expressed upon PRRSV infection and subsequently inhibits virus replication by reprogramming lipid droplet (LD) synthesis through transcriptional regulation. Our work provides a novel antiviral mechanism against PRRSV infection and suggests that targeting YY1 could be a new strategy for controlling PRRSV.
Keywords: antiviral; lipid droplet; porcine reproductive and respiratory syndrome virus; reprogram; yin yang 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xu K, Zhou Y, Mu Y, Liu Z, Hou S, Xiong Y, Fang L, Ge C, Wei Y, Zhang X, Xu C, Che J, Fan Z, Xiang G, Guo J, Shang H, Li H, Xiao S, Li J, Li K. 2020. CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance. eLife 9. doi:10.7554/eLife.57132 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32172846/MOST | National Natural Science Foundation of China (NSFC)
- 2022YFD1800304/MOST | National Key Research and Development Program of China (NKPs)
- CARS-35/MOA | Earmarked Fund for China Agriculture Research System
- 23JRRA1476/Joint Research Foundation of Gansu Province
- 23JRRA1480/Joint Research Foundation of Gansu Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

