Brincidofovir inhibits polyomavirus infection in vivo
- PMID: 38953354
- PMCID: PMC11323531
- DOI: 10.1128/mbio.01049-24
Brincidofovir inhibits polyomavirus infection in vivo
Abstract
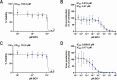
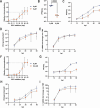
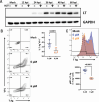
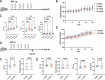
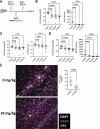
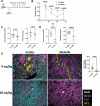
Polyomaviruses are species-specific DNA viruses that can cause disease in immunocompromised individuals. Despite their role as the causative agents for several diseases, there are no currently approved antivirals for treating polyomavirus infection. Brincidofovir (BCV) is an antiviral approved for the treatment of poxvirus infections and has shown activity against other double-stranded DNA viruses. In this study, we tested the efficacy of BCV against polyomavirus infection in vitro and in vivo using mouse polyomavirus (MuPyV). BCV inhibited virus production in primary mouse kidney cells and brain cortical cells. BCV treatment of cells transfected with MuPyV genomic DNA resulted in a reduction in virus levels, indicating that viral inhibition occurs post-entry. Although in vitro BCV treatment had a limited effect on viral DNA and RNA levels, drug treatment was associated with a reduction in viral protein, raising the possibility that BCV acts post-transcriptionally to inhibit MuPyV infection. In mice, BCV treatment was well tolerated, and prophylactic treatment resulted in a reduction in viral DNA levels and a potent suppression of infectious virus production in the kidney and brain. In mice with chronic polyomavirus infection, therapeutic administration of BCV decreased viremia and reduced infection in the kidney. These data demonstrate that BCV exerts antiviral activity against polyomavirus infection in vivo, supporting further investigation into the use of BCV to treat clinical polyomavirus infections.
Importance: Widespread in the human population and able to persist asymptomatically for the life of an individual, polyomavirus infections cause a significant disease burden in the immunocompromised. Individuals undergoing immune suppression, such as kidney transplant patients or those treated for autoimmune diseases, are particularly at high risk for polyomavirus-associated diseases. Because no antiviral agent exists for treating polyomavirus infections, management of polyomavirus-associated diseases typically involves reducing or discontinuing immunomodulatory therapy. This can be perilous due to the risk of transplant rejection and the potential development of adverse immune reactions. Thus, there is a pressing need for the development of antivirals targeting polyomaviruses. Here, we investigate the effects of brincidofovir, an FDA-approved antiviral, on polyomavirus infection in vivo using mouse polyomavirus. We show that the drug is well-tolerated in mice, reduces infectious viral titers, and limits viral pathology, indicating the potential of brincidofovir as an anti-polyomavirus therapeutic.
Keywords: antiviral agents; brain; brincidofovir; kidney; polyomavirus.
Conflict of interest statement
K.F. and M.H. are employees of Symbio, which owns the license to BCV.
Figures
Similar articles
-
Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model.mSphere. 2021 Feb 3;6(1):e00927-20. doi: 10.1128/mSphere.00927-20. mSphere. 2021. PMID: 33536322 Free PMC article.
-
Buccal viral DNA as a trigger for brincidofovir therapy in the mousepox model of smallpox.Antiviral Res. 2017 Mar;139:112-116. doi: 10.1016/j.antiviral.2016.12.015. Epub 2016 Dec 27. Antiviral Res. 2017. PMID: 28039021 Free PMC article.
-
Antiviral activity of brincidofovir on parvovirus B19.Antiviral Res. 2019 Feb;162:22-29. doi: 10.1016/j.antiviral.2018.12.003. Epub 2018 Dec 8. Antiviral Res. 2019. PMID: 30529090
-
Brincidofovir: understanding its unique profile and potential role against adenovirus and other viral infections.Future Microbiol. 2020 Apr;15:389-400. doi: 10.2217/fmb-2019-0288. Epub 2020 Mar 13. Future Microbiol. 2020. PMID: 32166967 Review.
-
Insights into the mechanism of action of cidofovir and other acyclic nucleoside phosphonates against polyoma- and papillomaviruses and non-viral induced neoplasia.Antiviral Res. 2015 Feb;114:21-46. doi: 10.1016/j.antiviral.2014.10.012. Epub 2014 Oct 30. Antiviral Res. 2015. PMID: 25446403 Review.
Cited by
-
GPCR Inhibitors Have Antiviral Properties against JC Polyomavirus Infection.Viruses. 2024 Sep 30;16(10):1559. doi: 10.3390/v16101559. Viruses. 2024. PMID: 39459893 Free PMC article.
References
-
- Buck CB, Van Doorslaer K, Peretti A, Geoghegan EM, Tisza MJ, An P, Katz JP, Pipas JM, McBride AA, Camus AC, McDermott AJ, Dill JA, Delwart E, Ng TFF, Farkas K, Austin C, Kraberger S, Davison W, Pastrana DV, Varsani A. 2016. The ancient evolutionary history of polyomaviruses. PLoS Pathog 12:e1005574. doi: 10.1371/journal.ppat.1005574 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

