Pneumocystis murina promotes inflammasome formation and NETosis during Pneumocystis pneumonia
- PMID: 38953359
- PMCID: PMC11323544
- DOI: 10.1128/mbio.01409-24
Pneumocystis murina promotes inflammasome formation and NETosis during Pneumocystis pneumonia
Abstract
Pneumocystis jirovecii pneumonia (PjP) poses a serious risk to individuals with compromised immune systems, such as individuals with HIV/AIDS or undergoing immunosuppressive therapies for cancer or solid organ transplants. Severe PjP triggers excessive lung inflammation, resulting in lung function decline and consequential alveolar damage, potentially culminating in acute respiratory distress syndrome. Non-HIV patients face a 30%-60% mortality rate, emphasizing the need for a deeper understanding of inflammatory responses in PjP. Prior research emphasized macrophages in Pneumocystis infections, neglecting neutrophils' role in tissue damage. Consequently, the overemphasis on macrophages led to an incomplete understanding of the role of neutrophils and inflammatory responses. In the current investigation, our RNAseq studies on a murine surrogate model of PjP revealed heightened activation of the NLRP3 inflammasome and NETosis cell death pathways in their lungs. Immunofluorescence staining confirmed neutrophil extracellular trap (NET) presence in the lungs of the P. murina-infected mice, validating our findings. Moreover, isolated neutrophils exhibited NETosis when directly stimulated with P. murina. Isolated NETs compromised P. murina viability in vitro, highlighting the potential role of neutrophils in controlling fungal growth and promoting inflammation during P. murina pneumonia through NLRP3 inflammasome assembly and NETosis. These pathways, essential for inflammation and pathogen elimination, bear the risk of uncontrolled activation leading to excessive tissue damage and persistent inflammation. This pioneering study is the first to identify the formation of NETs and inflammasomes during Pneumocystis infection, paving the way for comprehensive investigations into treatments aimed at mitigating lung damage and augmenting survival rates for individuals with PjP.IMPORTANCEPneumocystis jirovecii pneumonia (PjP) affects individuals with weakened immunity, such as HIV/AIDS, cancer, and organ transplant patients. Severe PjP triggers lung inflammation, impairing function and potentially causing acute respiratory distress syndrome. Non-HIV individuals face a 30%-60% mortality rate, underscoring the need for deeper insight into PjP's inflammatory responses. Past research focused on macrophages in managing Pneumocystis infection and its inflammation, while the role of neutrophils was generally overlooked. In contrast, our findings in P. murina-infected mouse lungs showed neutrophil involvement during inflammation and increased expression of NLRP3 inflammasome and NETosis pathways. Detection of neutrophil extracellular traps further indicated their involvement in the inflammatory process. Although beneficial in combating infection, unregulated neutrophil activation poses a potential threat to lung tissues. Understanding the behavior of neutrophils in Pneumocystis infections is crucial for controlling detrimental reactions and formulating treatments to reduce lung damage, ultimately improving the survival rates of individuals with PjP.
Keywords: NETosis; Pneumocystis; Pneumocystis murina; Pneumocystis pneumonia; immunity; infectious disease; inflammation; neutrophils; opportunistic fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
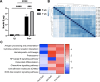
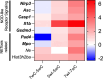
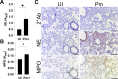


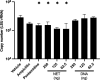
Update of
-
Pneumocystis murina Promotes Inflammasome Formation and NETosis during Pneumocystis Pneumonia.bioRxiv [Preprint]. 2024 Feb 18:2024.02.16.580773. doi: 10.1101/2024.02.16.580773. bioRxiv. 2024. Update in: mBio. 2024 Aug 14;15(8):e0140924. doi: 10.1128/mbio.01409-24. PMID: 38405901 Free PMC article. Updated. Preprint.
Similar articles
-
Pneumocystis murina Promotes Inflammasome Formation and NETosis during Pneumocystis Pneumonia.bioRxiv [Preprint]. 2024 Feb 18:2024.02.16.580773. doi: 10.1101/2024.02.16.580773. bioRxiv. 2024. Update in: mBio. 2024 Aug 14;15(8):e0140924. doi: 10.1128/mbio.01409-24. PMID: 38405901 Free PMC article. Updated. Preprint.
-
Sex-specific NLRP3 activation in neutrophils promotes neutrophil recruitment and NETosis in the murine model of diffuse alveolar hemorrhage.Front Immunol. 2024 Nov 25;15:1466234. doi: 10.3389/fimmu.2024.1466234. eCollection 2024. Front Immunol. 2024. PMID: 39654901 Free PMC article.
-
Progesterone suppresses rhinovirus-induced airway inflammation by inhibiting neutrophil infiltration and extracellular traps formation.Int Immunopharmacol. 2025 Jan 10;144:113714. doi: 10.1016/j.intimp.2024.113714. Epub 2024 Dec 2. Int Immunopharmacol. 2025. PMID: 39626540
-
Inflammasome Activation and Neutrophil Extracellular Traps in Atherosclerosis.J Atheroscler Thromb. 2025 May 1;32(5):535-549. doi: 10.5551/jat.RV22033. Epub 2025 Jan 18. J Atheroscler Thromb. 2025. PMID: 39828369 Free PMC article. Review.
-
Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population.Ann Pharmacother. 2016 Aug;50(8):673-9. doi: 10.1177/1060028016650107. Epub 2016 May 30. Ann Pharmacother. 2016. PMID: 27242349 Review.
Cited by
-
Individualized Trimethoprim-Sulfamethoxazole Dosing in Non-HIV Patients with Pneumocystis Pneumonia: A Narrative Review of Current Evidence.J Pers Med. 2025 Jul 14;15(7):311. doi: 10.3390/jpm15070311. J Pers Med. 2025. PMID: 40710428 Free PMC article. Review.
References
-
- Kolbrink B, Scheikholeslami-Sabzewari J, Borzikowsky C, von Samson-Himmelstjerna FA, Ullmann AJ, Kunzendorf U, Schulte K. 2022. Evolving epidemiology of Pneumocystis pneumonia: findings from a longitudinal population-based study and a retrospective multi-center study in Germany. Lancet Reg Health Eur 18:100400. doi:10.1016/j.lanepe.2022.100400 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

