Simultaneous quantitation of neutralizing antibodies against all four dengue virus serotypes using optimized reporter virus particles
- PMID: 38953379
- PMCID: PMC11265411
- DOI: 10.1128/jvi.00681-24
Simultaneous quantitation of neutralizing antibodies against all four dengue virus serotypes using optimized reporter virus particles
Abstract
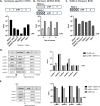
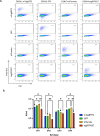
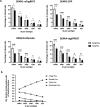
Serum-neutralizing antibody titers are a critical measure of vaccine immunogenicity and are used to determine flavivirus seroprevalence in study populations. An effective dengue virus (DENV) vaccine must confer simultaneous protection against viruses grouped within four antigenic serotypes. Existing flavivirus neutralization assays, including the commonly used plaque/focus reduction neutralization titer (PRNT/FRNT) assay, require an individual assay for each virus, serotype, and strain and easily become a labor-intensive and time-consuming effort for large epidemiological studies or vaccine trials. Here, we describe a multiplex reporter virus particle neutralization titer (TetraPlex RVPNT) assay for DENV that allows simultaneous quantitative measures of antibody-mediated neutralization of infection against all four DENV serotypes in a single low-volume clinical sample and analyzed by flow cytometry. Comparative studies confirm that the neutralization titers of antibodies measured by the TetraPlex RVPNT assay are similar to FRNT/PRNT assay approaches performed separately for each viral strain. The use of this high-throughput approach enables the careful serological study in DENV endemic populations and vaccine recipients required to support the development of a safe and effective tetravalent DENV vaccine.
Importance: As a mediator of protection against dengue disease and a serological indicator of prior infection, the detection and quantification of neutralizing antibodies against DENV is an important "gold standard" tool. However, execution of traditional neutralizing antibody assays is often cumbersome and requires repeated application for each virus or serotype. The optimized RVPNT assay described here is high-throughput, easily multiplexed across multiple serotypes, and targets reporter viral particles that can be robustly produced for all four DENV serotypes. The use of this transformative RVPNT assay will support the expansion of neutralizing antibody datasets to answer research and public health questions often limited by the more cumbersome neutralizing antibody assays and the need for greater quantities of test serum.
Keywords: dengue virus; flow cytometry; immunoassays; neutralizing antibodies; viral immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

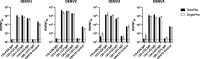

References
-
- Pierson TC, Diamond MS. 2013. Flaviviruses, p 747–794. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR (ed), Fields virology, 6th ed, Vol. 1. Lippincott Williams&Wilkins, Philadelphia.
-
- Rivera L, Biswal S, Sáez-Llorens X, Reynales H, López-Medina E, Borja-Tabora C, Bravo L, Sirivichayakul C, Kosalaraksa P, Martinez Vargas L, et al. . 2022. Three-year efficacy and safety of Takeda’s dengue vaccine candidate (TAK-003). Clin Infect Dis 75:107–117. doi:10.1093/cid/ciab864 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

