Hydroxyurea to prevent brain injury in children with sickle cell disease (HU Prevent)-A randomized, placebo-controlled phase II feasibility/pilot study
- PMID: 38953438
- PMCID: PMC11502276
- DOI: 10.1002/ajh.27423
Hydroxyurea to prevent brain injury in children with sickle cell disease (HU Prevent)-A randomized, placebo-controlled phase II feasibility/pilot study
Abstract
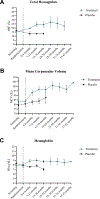
Central nervous system (CNS) injury is common in sickle cell disease (SCD) and occurs early in life. Hydroxyurea is safe and efficacious for treatment of SCD, but high-quality evidence from randomized trials to estimate its neuroprotective effect is scant. HU Prevent was a randomized (1:1), double-blind, phase II feasibility/pilot trial of dose-escalated hydroxyurea vs. placebo for the primary prevention of CNS injury in children with HbSS or HbS-β0-thalassemia subtypes of SCD age 12-48 months with normal neurological examination, MRI of the brain, and cerebral blood flow velocity. We hypothesized that hydroxyurea would reduce by 50% the incidence of CNS injury. Two outcomes were compared: primary-a composite of silent cerebral infarction, elevated cerebral blood flow velocity, transient ischemic attack, or stroke; secondary-a weighted score estimating the risk of suffering the consequences of stroke (the Stroke Consequences Risk Score-SCRS), based on the same outcome events. Six participants were randomized to each group. One participant in the hydroxyurea group had a primary outcome vs. four in the placebo group (incidence rate ratio [90% CI] 0.216 [0.009, 1.66], p = .2914) (~80% reduction in the hydroxyurea group). The mean SCRS score was 0.078 (SD 0.174) in the hydroxyurea group, 0.312 (SD 0.174) in the placebo group, p = .072, below the p-value of .10 often used to justify subsequent phase III investigations. Serious adverse events related to study procedures occurred in 3/41 MRIs performed, all related to sedation. These results suggest that hydroxyurea may have profound neuroprotective effect in children with SCD and support a definitive phase III study to encourage the early use of hydroxyurea in all infants with SCD.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
JFC reports funding from the NIH for the current study, as well as other NIH, HRSA, and CDC funding; invention, patent and a licensing agreement to ImmunArray through Johns Hopkins for a panel of brain biomarkers for the detection of brain injury; and invention and patent of aptamers as a potential treatment for sickle cell disease. RJA reports consultancy to a pharmaceutic company (Global Blood Therapeutics, now a wholly-owned subsidiary of Pfizer) doing research on stroke in sickle cell disease, unrelated to hydroxyurea. DJB reports support from his employer’s (RTI International) overhead funds for time invested in this study and manuscript as enhancement of his scientific stature. JDL reports consultant roles with Agios Pharmaceuticals, Novartis, and Editas. LCJ reports minor royalties as author of a chapter in UpToDate on prevention of stroke in sickle cell disease. AAK reports grant funding from the NIH for this study. EAB-C reports a patent of aptamers as a potential treatment for sickle cell disease; and the same conflicts as JFC, who is her spouse. CKE reports support for provision of research support services as a Faculty Lead with the Johns Hopkins Biostatistics, Epidemiology, And Data Management (BEAD) Core. JJS reports funding from the NIH for the current study; royalties from UpToDate for authorship of “Hydroxyurea for the Treatment of Sickle Cell Disease”; payment as topic editor for Dynamed, “Sickle Cell Disease in Infants and Children”; and participation on a Disc Medicine scientific advisory board for design of a phase 1 study of biopterin for sickle cell disease. DFH reports NIH funding paid to JHU for the current study; grant funding from the Department of Defense; medicolegal consulting; consulting fees paid to him from Synaptogenix/Neurotrope and Neurelis and stockholding in EpiWatch.
DKF, JJF, RNI, RCM, MAK, DWS, DAW, DAW-S, RA, ADC, KAR, JES, CAS, DCW, NDO, KV, KET, CEK, and RET report no conflicts of interest.
Figures
References
-
- Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Mental Retardation and Developmental Disabilities Reviews 2006; 12: 236–45. - PubMed
-
- Ohene-Frempong K, Weiner SJ, Sleeper L a, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998; 91: 288–94. - PubMed
-
- Runge A, Brazel D, Pakbaz Z. Stroke in sickle cell disease and the promise of recent disease modifying agents. J Neurol Sci 2022; 442: 120412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
