Identification of Innovative Folate Inhibitors Leveraging the Amino Dihydrotriazine Motif from Cycloguanil for Their Potential as Anti- Trypanosoma brucei Agents
- PMID: 38953453
- PMCID: PMC11537224
- DOI: 10.1021/acsinfecdis.4c00113
Identification of Innovative Folate Inhibitors Leveraging the Amino Dihydrotriazine Motif from Cycloguanil for Their Potential as Anti- Trypanosoma brucei Agents
Abstract
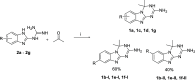
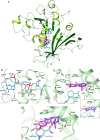
Folate enzymes, namely, dihydrofolate reductase (DHFR) and pteridine reductase (PTR1) are acknowledged targets for the development of antiparasitic agents against Trypanosomiasis and Leishmaniasis. Based on the amino dihydrotriazine motif of the drug Cycloguanil (Cyc), a known inhibitor of both folate enzymes, we have identified two novel series of inhibitors, the 2-amino triazino benzimidazoles (1) and 2-guanidino benzimidazoles (2), as their open ring analogues. Enzymatic screening was carried out against PTR1, DHFR, and thymidylate synthase (TS). The crystal structures of TbDHFR and TbPTR1 in complex with selected compounds experienced in both cases a substrate-like binding mode and allowed the rationalization of the main chemical features supporting the inhibitor ability to target folate enzymes. Biological evaluation of both series was performed against T. brucei and L. infantum and the toxicity against THP-1 human macrophages. Notably, the 5,6-dimethyl-2-guanidinobenzimidazole 2g resulted to be the most potent (Ki = 9 nM) and highly selective TbDHFR inhibitor, 6000-fold over TbPTR1 and 394-fold over hDHFR. The 5,6-dimethyl tricyclic analogue 1g, despite showing a lower potency and selectivity profile than 2g, shared a comparable antiparasitic activity against T. brucei in the low micromolar domain. The dichloro-substituted 2-guanidino benzimidazoles 2c and 2d revealed their potent and broad-spectrum antitrypanosomatid activity affecting the growth of T. brucei and L. infantum parasites. Therefore, both chemotypes could represent promising templates that could be valorized for further drug development.
Keywords: Leishmania infantum; Trypanosoma brucei; antiparasitic agents; dihydrofolate reductase inhibitors; pteridine reductase inhibitors; triazino and guanidino benzimidazoles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Structural Insights into the Development of Cycloguanil Derivatives as Trypanosoma brucei Pteridine-Reductase-1 Inhibitors.ACS Infect Dis. 2019 Jul 12;5(7):1105-1114. doi: 10.1021/acsinfecdis.8b00358. Epub 2019 May 1. ACS Infect Dis. 2019. PMID: 31012301
-
Sesquiterpene Lactones with Dual Inhibitory Activity against the Trypanosoma brucei Pteridine Reductase 1 and Dihydrofolate Reductase.Molecules. 2021 Dec 27;27(1):149. doi: 10.3390/molecules27010149. Molecules. 2021. PMID: 35011381 Free PMC article.
-
Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development.Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1448-53. doi: 10.1073/pnas.0704384105. Epub 2008 Feb 1. Proc Natl Acad Sci U S A. 2008. PMID: 18245389 Free PMC article.
-
Targeting pteridine reductase 1 and dihydrofolate reductase: the old is a new trend for leishmaniasis drug discovery.Future Med Chem. 2019 Aug;11(16):2107-2130. doi: 10.4155/fmc-2018-0512. Epub 2019 Aug 2. Future Med Chem. 2019. PMID: 31370699 Review.
-
To Explore Potential Inhibitors against Various Enzymatic Targets of Human African Trypanosomiasis.Comb Chem High Throughput Screen. 2025;28(9):1553-1593. doi: 10.2174/0113862073293708240416113543. Comb Chem High Throughput Screen. 2025. PMID: 38676500 Review.
Cited by
-
2-Guanidinobenzimidazole as Ligand in Supramolecular, Coordination and Organometallic Chemistry.Int J Mol Sci. 2025 Jan 26;26(3):1063. doi: 10.3390/ijms26031063. Int J Mol Sci. 2025. PMID: 39940830 Free PMC article. Review.
-
Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques.Vet Sci. 2025 Jun 5;12(6):550. doi: 10.3390/vetsci12060550. Vet Sci. 2025. PMID: 40559787 Free PMC article.
References
-
- Neglected tropical diseases -- GLOBAL. https://www.who.int/health-topics/neglected-tropical-diseases (accessed May 2023).
-
- Trypanosomiasis, human African (sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-a... (accessed May 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

