Thiourea-functionalized aminoglutethimide derivatives as anti-leishmanial agents
- PMID: 38953461
- PMCID: PMC11370960
- DOI: 10.1080/17568919.2024.2359362
Thiourea-functionalized aminoglutethimide derivatives as anti-leishmanial agents
Abstract
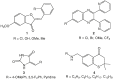
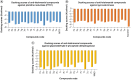
Aim: We aim to develop new anti-leishmanial agents against Leishmania major and Leishmania tropica.Materials & methods: A total of 23 thiourea derivatives of (±)-aminoglutethimide were synthesized and evaluated for in vitro activity against promastigotes of L. major and L. tropica.Results & conclusion: The N-benzoyl analogue 7p was found potent (IC50 = 12.7 μM) against L. major and non toxic to normal cells. The docking studies, indicates that these inhibitors may target folate and glycolytic pathways of the parasite. The N-hexyl compound 7v was found strongly active against both species, and lacked cytotoxicity against normal cells, whereas compound 7r, with a 3,5-bis-(tri-fluoro-methyl)phenyl unit, was active against Leishmania, but was cytotoxic in nature. Compound 7v was thus identified as a hit for further studies.
Keywords: (±)-Aminoglutethimide; cutaneous leishmaniasis; cytotoxicity; thiourea.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures



References
-
- Pawar S, Kumawat MK, Kundu M, et al. Synthetic and medicinal perspective of anti-leishmanial agents: an overview. J Mol Molecular Struct. 2023;1271:133977. doi: 10.1016/j.molstruc.2022.133977 - DOI
-
- Cecilio P, Cordeiro-da-Silva A, Oliveira F. Sand flies: basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol. 2022;5(1):305. doi: 10.1038/s42003-022-03240-z - DOI - PMC - PubMed
-
• Provides the basic information of vector of Leishmania and its interaction with Leishmanial parasite.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
