Efficient characterization of multiple binding sites of small molecule imaging ligands on amyloid-beta, tau and alpha-synuclein
- PMID: 38953933
- PMCID: PMC11527973
- DOI: 10.1007/s00259-024-06806-7
Efficient characterization of multiple binding sites of small molecule imaging ligands on amyloid-beta, tau and alpha-synuclein
Abstract
Purpose: There is an unmet need for compounds to detect fibrillar forms of alpha-synuclein (αSyn) and 4-repeat tau, which are critical in many neurodegenerative diseases. Here, we aim to develop an efficient surface plasmon resonance (SPR)-based assay to facilitate the characterization of small molecules that can bind these fibrils.
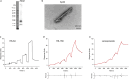
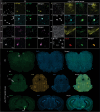
Methods: SPR measurements were conducted to characterize the binding properties of fluorescent ligands/compounds toward recombinant amyloid-beta (Aβ)42, K18-tau, full-length 2N4R-tau and αSyn fibrils. In silico modeling was performed to examine the binding pockets of ligands on αSyn fibrils. Immunofluorescence staining of postmortem brain tissue slices from Parkinson's disease patients and mouse models was performed with fluorescence ligands and specific antibodies.
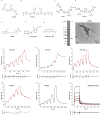
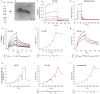
Results: We optimized the protocol for the immobilization of Aβ42, K18-tau, full-length 2N4R-tau and αSyn fibrils in a controlled aggregation state on SPR-sensor chips and for assessing their binding to ligands. The SPR results from the analysis of binding kinetics suggested the presence of at least two binding sites for all fibrils, including luminescent conjugated oligothiophenes, benzothiazole derivatives, nonfluorescent methylene blue and lansoprazole. In silico modeling studies for αSyn (6H6B) revealed four binding sites with a preference for one site on the surface. Immunofluorescence staining validated the detection of pS129-αSyn positivity in the brains of Parkinson's disease patients and αSyn preformed-fibril injected mice, 6E10-positive Aβ in arcAβ mice, and AT-8/AT-100-positivity in pR5 mice.
Conclusion: SPR measurements of small molecules binding to Aβ42, K18/full-length 2N4R-tau and αSyn fibrils suggested the existence of multiple binding sites. This approach may provide efficient characterization of compounds for neurodegenerative disease-relevant proteinopathies.
Keywords: Alpha-synuclein; Amyloid-beta; Binding sites; In silico; Surface plasmon resonance; Tau.
© 2024. The Author(s).
Conflict of interest statement
KS and RN are associate editors in Eur J Nucl Med Mol Imaging. RMN is an employee and shareholder of Neurimmune AG, Switzerland. The other authors declare no conflicts of interest.
Figures


References
-
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–22. 10.1016/S1474-4422(13)70090-5. - PubMed
-
- Smith R, Capotosti F, Schain M, Ohlsson T, Touilloux T, Hliva V, et al. Initial clinical scans using [18F]ACI-12589, a novel α-synuclein PET-tracer. Alzheimers Dement. 2022;18:e065394. 10.1002/alz.065394.

