Long-term responders to nivolumab in previously treated advanced renal cell carcinoma: a sub-analysis of meet-URO15 study
- PMID: 38954006
- PMCID: PMC11219688
- DOI: 10.1007/s00262-024-03741-2
Long-term responders to nivolumab in previously treated advanced renal cell carcinoma: a sub-analysis of meet-URO15 study
Abstract
Background: Although nivolumab prolongs overall survival (OS) in pretreated patients with metastatic renal cell carcinoma (mRCC), underlining clinical and biological features of long-term responses are still to be determined. This study aims to investigate clinical and pathological characteristics of mRCC patients who achieved long-term responses during nivolumab treatment.
Materials and methods: A retrospective analysis was performed on mRCC patients receiving nivolumab as second or further therapy line between May 2016 and January 2019 in 34 Italian Oncology Centres. Outcome assessments and logistic regression were performed to evaluate factors influencing long-term responses.
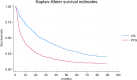
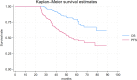
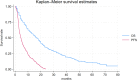
Results: A total of 571 patients with a median age of 61 years (range 17-85) were included in the analysis. With a median follow-up of 22.1 (1.0-89.0) months, 23.1% of patients were 2-year progression-free on treatment with nivolumab, hence they were categorized as long-term responders. Baseline characteristics, including age, gender, and histology, were similar between long- and short-term responders. Karnofsky Performance Status ≥ 80% was significantly associated with long-term response (p = 0.02), while bone metastases (p = 0.03), International mRCC Database Consortium intermediate-poor risk (p < 0.01) and Neutrophil-to-Lymphocyte Ratio ≥ 3.2 (p = 0.02) were associate with short-term responses. Long-term responders exhibited a median progression-free survival of 55.0 months versus 4.0 months of the short-term responders. The median OS was not reached in long-term responders while it was 17.0 months for short*term responders.
Conclusion: This retrospective analysis sheds light on factors associated with long-term response to nivolumab in mRCC. Understanding these clinical features will be essential for selecting patients who may mostly benefit from immunotherapy.
Keywords: Immunotherapy; Long-term response; Metastatic renal cell carcinoma; Nivolumab; Prognostic factors.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Rini BI, Powles T, Atkins MB et al (2019) Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393:2404–2415. 10.1016/S0140-6736(19)30723-8 10.1016/S0140-6736(19)30723-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical