Engagement in Care, Awareness, and Interest in Long-Acting Injectable Anti-Retroviral Therapy
- PMID: 38954172
- PMCID: PMC11427500
- DOI: 10.1007/s10461-024-04423-x
Engagement in Care, Awareness, and Interest in Long-Acting Injectable Anti-Retroviral Therapy
Abstract
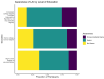
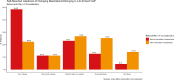
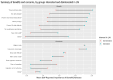
Long Acting Injectable (LAI) therapy to treat HIV is an alternative to daily oral medications. The success of early roll-out of LAI to eligible patients requires a better understanding of patients' awareness and interest in this novel therapy. We administered an electronic survey to patients attending an urban HIV clinic in the US South. Eligible participants were 18 + years old with a most recent HIV-1 viral load < 200 copies/ml, without any evidence of genotypic resistance to LAI components or chronic hepatitis B. Survey recipients were asked about current treatment, engagement in care, and knowledge of LAI. Between January-April 2023, 480 patients were screened; 319 were eligible, and 155 (49%) completed the survey. The majority (119, 77%) were aware of, and 87 (56%) were interested in LAI. In regression analysis, only age was associated with interest in LAI (OR 0.95, 95% CI 0.92,0.99). Among proposed benefits of injectables, ease of travel without pills, lack of daily pill-taking, and fewer medication interactions were most appealing. Among proposed concerns with injectables, higher cost and insurance coverage of the new medicine were most worrisome. A large majority of people with HIV (PWH) are aware of the newest treatment available, and just over half of our sample expressed interest in LAI. Older age was associated with lower interest in LAI. LAI is appealing for its convenience, privacy, and avoidance of drug interactions, while the increased costs associated with LAI need to be addressed.
Keywords: Anti-retroviral therapy; HIV; Long-acting injectables; Treatment preferences.
© 2024. The Author(s).
Conflict of interest statement
JAS, MA, FH, CC, KL, JS have no disclosures to report. MSM receives institutional funding from Gilead Sciences. CM is supported by a grant from the NHLBI, an institute of the NIH (K01HL159052).
Figures
References
-
- Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet Dec. 2021;19(10267):1994–2005. - PubMed
-
- Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV Nov. 2021;8(11):e679–89. - PubMed
-
- CAB + RPV Prescribing Info. In. Healthcare V, editor: Food and Drug Administration; 2022.
-
- Biello KB, Hosek S, Drucker MT, et al. Preferences for Injectable PrEP among Young U.S. Cisgender men and Transgender women and men who have sex with men. Arch Sex Behav Oct. 2018;47(7):2101–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

