Durability of Evoked Compound Action Potential (ECAP)-Controlled, Closed-Loop Spinal Cord Stimulation (SCS) in a Real-World European Chronic Pain Population
- PMID: 38954217
- PMCID: PMC11393244
- DOI: 10.1007/s40122-024-00628-z
Durability of Evoked Compound Action Potential (ECAP)-Controlled, Closed-Loop Spinal Cord Stimulation (SCS) in a Real-World European Chronic Pain Population
Abstract
Introduction: Closed-loop spinal cord stimulation (CL-SCS) is a recently introduced system that records evoked compound action potentials (ECAPs) from the spinal cord elicited by each stimulation pulse and uses this information to automatically adjust the stimulation strength in real time, known as ECAP-controlled SCS. This innovative system compensates for fluctuations in the distance between the epidural leads and the spinal cord by maintaining the neural response (ECAP) at a predetermined target level. This data collection study was designed to assess the performance of the first CL-SCS system in a real-world setting under normal conditions of use in multiple European centers. The study analyzes and presents clinical outcomes and electrophysiological and device data and compares these findings with those reported in earlier pre-market studies of the same system.
Methods: This prospective, multicenter, observational study was conducted in 13 European centers and aimed to gather electrophysiological and device data. The study focused on the real-world application of this system in treating chronic pain affecting the trunk and/or limbs, adhering to standard conditions of use. In addition to collecting and analyzing basic demographic information, the study presents data from the inaugural patient cohort permanently implanted at multiple European centers.
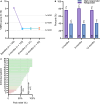
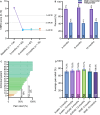
Results: A significant decrease in pain intensity was observed for overall back or leg pain scores (verbal numerical rating score [VNRS]) between baseline (mean ± standard error of the mean [SEM]; n = 135; 8.2 ± 0.1), 3 months (n = 93; 2.3 ± 0.2), 6 months (n = 82; 2.5 ± 0.3), and 12 months (n = 76; 2.5 ± 0.3). Comparison of overall pain relief (%) to the AVALON and EVOKE studies showed no significant differences at 3 and 12 months between the real-world data release (RWE; 71.3%; 69.6%) and the AVALON (71.2%; 73.6%) and EVOKE (78.1%; 76.7%) studies. Further investigation was undertaken to objectively characterize the physiological parameters of SCS therapy in this cohort using the metrics of percent time above ECAP threshold (%), dose ratio, and dose accuracy (µV), according to previously described methods. Results showed that a median of 90% (40.7-99.2) of stimuli were above the ECAP threshold, with a dose ratio of 1.3 (1.1-1.4) and dose accuracy of 4.4 µV (0.0-7.1), based on data from 236, 230, and 254 patients, respectively. Thus, across all three metrics, the majority of patients had objective therapy metrics corresponding to the highest levels of pain relief in previously reported studies (usage over threshold > 80%, dose ratio > 1.2, and error < 10 µV).
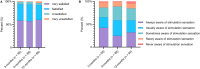
Conclusions: In conclusion, this study provides valuable insights into the real-world application of the ECAP-controlled CL-SCS system, highlighting its potential for maintaining effective pain relief and objective neurophysiological therapy metrics at levels seen in randomized control trials, and potential for quantifying patient burden associated with SCS system use via patient-device interaction metrics.
Clinical trial registration: In the Netherlands, the study is duly registered on the International Clinical Trials Registry Platform (Trial NL7889). In Germany, the study is duly registered as NCT05272137 and in the United Kingdom as ISCRTN27710516 and has been reviewed by the ethics committee in both countries.
Keywords: Chronic; Closed-loop; ECAP-controlled closed-loop; Electrophysiology; Evoked compound action potential; Neuropathic pain; Pain; Physiologic closed-loop control; Spinal cord stimulation.
© 2024. The Author(s).
Conflict of interest statement
Harold Nijhuis is a paid consultant for Saluda Medical, and Abbott. Harold Nijhuis has a research grant with Abbott. Willem-Jan Hofsté is a paid consultant for Saluda Medical and Abbott. Philippa Armstrong has received travel grants and speaker fees from Saluda Medical. Johan van de Minkelis is a paid consultant for Saluda Medical. Jan Vesper is a paid consultant for Abbott, Boston Scientific, UniQure, Curonix. Jan Vesper has research grants with Abbott and Boston Scientific and is on the advisory board of Abbott. Ashish Gulve is on the advisory board for Medtronic and Boston Scientific. Ashish Gulve has a research grant with Saluda Medical. Ashish Gulve reports personal fees unrelated to the submitted work from Nevro, Medtronic, Boston Scientific and Mainstay Medical. Jan-Willem Kallewaard is on the advisory board of Saluda Medical, Medtronic, Boston Scientific and Abbott. Jan-Willem Kallewaard has research grants from Boston Scientific, Abbott, Nevro and Saluda Medical. Frank Huygen reports personal fees from Abbott, grants, personal fees from Saluda, personal fees from Boston Scientific, personal fees from Grunenthal, personal fees from Pfizer, outside the submitted work. Serge Nikolic is a paid consultant for Saluda Medical, Nevro, and Stratus Medical. Birte E. Dietz and Dave Mugan are employed by Saluda Medical. There are no other relationships that might lead to a conflict of interest in the current study. All other authors have no conflicts of interest to declare.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Medical

