Final Results From a Phase I Trial and Expansion Cohorts of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced/Metastatic Genitourinary Tumors
- PMID: 38954785
- PMCID: PMC11361361
- DOI: 10.1200/JCO.23.02233
Final Results From a Phase I Trial and Expansion Cohorts of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced/Metastatic Genitourinary Tumors
Abstract
Purpose: Cabozantinib and nivolumab (CaboNivo) alone or with ipilimumab (CaboNivoIpi) have shown promising efficacy and safety in patients with metastatic urothelial carcinoma (mUC), metastatic renal cell carcinoma (mRCC), and rare genitourinary (GU) tumors in a dose-escalation phase I study. We report the final data analysis of the safety, overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) of the phase I patients and seven expansion cohorts.
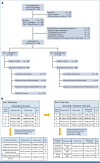
Methods: This is an investigator-initiated, multicenter, phase I trial. CaboNivo doublet expansion cohorts included (1) mUC, (2) mRCC, and (3) adenocarcinoma of the bladder/urachal; CaboNivoIpi triplet expansion cohorts included (1) mUC, (2) mRCC, (3) penile cancer, and (4) squamous cell carcinoma of the bladder and other rare GU tumors (ClinicalTrials.gov identifier: NCT02496208).
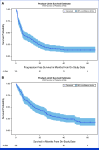
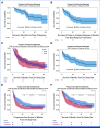
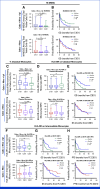
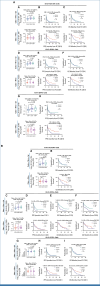
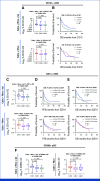
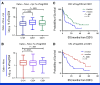
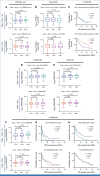
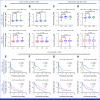
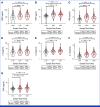
Results: The study enrolled 120 patients treated with CaboNivo (n = 64) or CaboNivoIpi (n = 56), with a median follow-up of 49.2 months. In 108 evaluable patients (CaboNivo n = 59; CaboNivoIpi n = 49), the ORR was 38% (complete response rate 11%) and the median duration of response was 20 months. The ORR was 42.4% for mUC, 62.5% for mRCC (n = 16), 85.7% for squamous cell carcinoma of the bladder (n = 7), 44.4% for penile cancer (n = 9), and 50.0% for renal medullary carcinoma (n = 2). Grade ≥ 3 treatment-related adverse events occurred in 84% of CaboNivo patients and 80% of CaboNivoIpi patients.
Conclusion: CaboNivo and CaboNivoIpi demonstrated clinical activity and safety in patients with multiple GU malignancies, especially clear cell RCC, urothelial carcinoma, and rare GU tumors such as squamous cell carcinoma of the bladder, small cell carcinoma of the bladder, adenocarcinoma of the bladder, renal medullary carcinoma, and penile cancer.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, et al. : Cancer statistics, 2023. CA Cancer J Clin 73:17-48, 2023 - PubMed
-
- Nadal R, Bellmunt J: Management of metastatic bladder cancer. Cancer Treat Rev 76:10-21, 2019 - PubMed
-
- de Velasco G, Bex A, Albiges L, et al. : Sequencing and combination of systemic therapy in metastatic renal cell carcinoma. Eur Urol Oncol 2:505-514, 2019 - PubMed
-
- Aragon-Ching JB, Pagliaro LC: New developments and challenges in rare genitourinary tumors: Non-urothelial bladder cancers and squamous cell cancers of the penis. Am Soc Clin Oncol Educ Book 37:330-336, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

