In vivo detection of Alzheimer's and Lewy body disease concurrence: Clinical implications and future perspectives
- PMID: 38955137
- PMCID: PMC11350051
- DOI: 10.1002/alz.14039
In vivo detection of Alzheimer's and Lewy body disease concurrence: Clinical implications and future perspectives
Abstract
Introduction: The recent introduction of seed amplification assays (SAAs) detecting misfolded α-synuclein, a pathology-specific marker for Lewy body disease (LBD), has allowed the in vivo identification and phenotypic characterization of patients with co-occurring Alzheimer's disease (AD) and LBD since the early clinical or even preclinical stage.
Methods: We reviewed studies with an in vivo biomarker-based diagnosis of AD-LBD copathology.
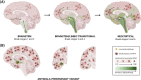
Results: Studies in large cohorts of cognitively impaired individuals have shown that cerebrospinal fluid (CSF) biomarkers detect the coexistence of AD and LB pathology in approximately 20%-25% of them, independently of the primary clinical diagnosis. Compared to those with pure AD, AD-LBD patients showed worse global cognition, especially in attentive/executive and visuospatial functions, and worse motor functions. In cognitively unimpaired individuals, concurrent AD-LBD pathologies predicted longitudinal cognitive progression with faster worsening of global cognition, memory, and attentive/executive functions.
Discussion: Future research studies aiming for a better precision medicine approach should develop SAAs further to reach a quantitative evaluation or staging of each underlying pathology using a single biofluid sample.
Highlights: α-Synuclein seed amplification assays (SAAs) provide a specific marker for Lewy body disease (LBD). SAAs allow for the in vivo identification of co-occurring LBD in patients with Alzheimer's disease (AD). AD-LBD coexist in 20-25% of cognitively impaired elderly individuals, and ∼8% of those asymptomatic. Compared to pure AD, AD-LBD causes a faster worsening of cognitive functions. AD-LBD is associated with worse attentive/executive, memory, visuospatial and motor functions.
Keywords: Lewy body disease; RT‐QuIC; dementia; prions; real‐time quaking‐induced conversion; synuclein.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare that they have no competing interests. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- PE0000006/NextGenerationEU (NGEU), Ministry of the University and Research (MUR), National Recovery and Resilience Plan (NRRP)
- 2022-00775/Work at Lund University was funded by Swedish Research Council
- ERAPERMED2021-184/ERA PerMed
- 2022-0231/Knut and Alice Wallenberg foundation
- Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's disease) at Lund University
- AF-980907/Swedish Alzheimer Foundation
- FO2021-0293/Swedish Brain Foundation
- Parkinson foundation of Sweden/Swedish Brain Foundation
- 1412/22/Swedish Brain Foundation
- Cure Alzheimer's fund, Rönström Family Foundation
- Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse
- 2020-O000028/Skåne University Hospital Foundation
- 2022-1259/Regionalt Forskningsstöd
- 2022-Projekt0080/Swedish federal government under the ALF agreement
- "Ricerca Corrente" funding from the Italian Ministry of Health
LinkOut - more resources
Full Text Sources
Medical

