Pancreatic STAT5 activation promotes KrasG12D-induced and inflammation-induced acinar-to-ductal metaplasia and pancreatic cancer
- PMID: 38955401
- PMCID: PMC11503187
- DOI: 10.1136/gutjnl-2024-332225
Pancreatic STAT5 activation promotes KrasG12D-induced and inflammation-induced acinar-to-ductal metaplasia and pancreatic cancer
Erratum in
-
Correction: pancreatic STAT5 activation promotes KrasG12D-induced and inflammation-induced acinar-to-ductal metaplasia and pancreatic cancer.Gut. 2025 Jul 7;74(8):e17. doi: 10.1136/gutjnl-2024-332225corr1. Gut. 2025. PMID: 40602997 No abstract available.
Abstract
Objective: Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy because it is often diagnosed at a late-stage. Signal transducer and activator of transcription 5 (STAT5) is a transcription factor implicated in the progression of various cancer types. However, its role in KRAS-driven pancreatic tumourigenesis remains unclear.
Design: We performed studies with LSL-Kras G12D; Ptf1a-Cre ERT (KCERT) mice or LSL-KrasG12D; LSL-Trp53R172H ; Pdx1-Cre (KPC) mice crossed with conditional disruption of STAT5 or completed deficiency interleukin (IL)-22. Pancreatitis was induced in mice by administration of cerulein. Pharmacological inhibition of STAT5 on PDAC prevention was studied in the orthotopic transplantation and patient-derived xenografts PDAC model, and KPC mice.
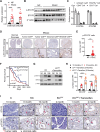
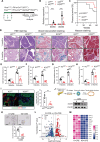
Results: The expression and phosphorylation of STAT5 were higher in human PDAC samples than control samples and high levels of STAT5 in tumour cells were associated with a poorer prognosis. The loss of STAT5 in pancreatic cells substantially reduces the KRAS mutation and pancreatitis-derived acinar-to-ductal metaplasia (ADM) and PDAC lesions. Mechanistically, we discovered that STAT5 binds directly to the promoters of ADM mediators, hepatocyte nuclear factor (HNF) 1β and HNF4α. Furthermore, STAT5 plays a crucial role in maintaining energy metabolism in tumour cells during PDAC progression. IL-22 signalling induced by chronic inflammation enhances KRAS-mutant-mediated STAT5 phosphorylation. Deficiency of IL-22 signalling slowed the progression of PDAC and ablated STAT5 activation.
Conclusion: Collectively, our findings identified pancreatic STAT5 activation as a key downstream effector of oncogenic KRAS signalling that is critical for ADM initiation and PDAC progression, highlighting its potential therapeutic vulnerability.
Keywords: ENERGY METABOLISM; INFLAMMATION; PANCREATIC CANCER.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
