Safety and Performance of a Cell-Impermeable Endoprosthesis for Hemodialysis Vascular Access Outflow Stenosis: A Brazilian Multicenter Retrospective Study
- PMID: 38955816
- PMCID: PMC11303476
- DOI: 10.1007/s00270-024-03790-1
Safety and Performance of a Cell-Impermeable Endoprosthesis for Hemodialysis Vascular Access Outflow Stenosis: A Brazilian Multicenter Retrospective Study
Abstract
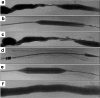
Purpose: To evaluate the safety and performance of Wrapsody™, a cell-impermeable endoprosthesis (CIE), for treating hemodialysis vascular access outflow stenosis.
Materials and methods: Investigators retrospectively analyzed 113 hemodialysis patients treated with a CIE (11/2021-12/2022) across four centers in Brazil. De novo or restenotic lesions were treated. The primary efficacy outcome measure was target lesion primary patency (TLPP) at 1, 3, 6, and 12 months; the primary safety outcome measure was the absence of serious local or systemic adverse events within the first 30 days post-procedure. Secondary outcome measures included technical and procedural success, access circuit primary patency (ACPP), and secondary patency at 1, 3, 6, and 12 months post-procedure.
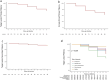
Results: Thirty-nine patients (34.5%) had thrombosed access at the initial presentation, and 38 patients (33.6%) presented with recurrent stenosis. TLPP rates at 1, 3, 6, and 12 months were 100%, 96.4%, 86.4%, and 69.7%, respectively. ACPP rates were 100% at 1 month, 89.2% at 3 months, 70.9% at 6 months, and 56.0% at 12 months. The target lesion secondary patency rates at 1, 3, 6, and 12 months were 100%, 97.3%, 93.6%, and 91.7%, respectively. In the adjusted multivariate Cox regression analysis, male sex and endoprosthesis with diameters of 10, 12, 14, and 16 mm were associated with improved primary patency rates. No localized or systemic serious adverse event was observed through the first 30 days post-procedure.
Conclusion: The CIE evaluated in this study is safe and effective for treating peripheral and central outflow stenoses in hemodialysis vascular access.
Level of evidence: Level 2b, cohort study.
Keywords: Cell-impermeable endoprosthesis; Covered stent; Hemodialysis; Stenosis; Stent graft; Vascular access.
© 2024. The Author(s).
Conflict of interest statement
L.O.H. reports receipt of research grants from Merit Medical Systems and Bard BD and consulting or lecture fees from Medtronic, Bard BD, Merit Medical Systems, Scitech Medical, and Gore. T.A.B., M.G.F., A.M.G., and L.C.A. report receiving research grants from Merit Medical Systems, Scitech Medical©, and Bard BD, as well as consulting and lecture fees from Medtronic, Bard BD, Merit Medical Systems, Inc., Scitech Medical, and Gore. J.P.S.M. received consultancy fees from Fresenius Medical Care Brazil. J.B.G. Nothing to disclose. B.R.V. Nothing to disclose. R.S.M. Nothing to disclose.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

