Characterizing Enoxaparin's Population Pharmacokinetics to Guide Dose Individualization in the Pediatric Population
- PMID: 38955947
- PMCID: PMC11288483
- DOI: 10.1007/s40262-024-01388-x
Characterizing Enoxaparin's Population Pharmacokinetics to Guide Dose Individualization in the Pediatric Population
Abstract
Background and objective: Pediatric dosing of enoxaparin was derived based on extrapolation of the adult therapeutic range to children. However, a large fraction of children do not achieve therapeutic anticoagulation with initial dosing. We aim to use real-world anti-Xa data obtained from children receiving enoxaparin per standard of care to characterize the population pharmacokinetics (PopPK).Author names: Please confirm if the author names are presented accurately and in the correct sequence (given name, middle name/initial, family name). Also, kindly confirm the details in the metadata are correct.The author names are accurately presented and the metadata are correct. METHODS: A PopPK analysis was performed using NONMEM, and a stepwise covariate modeling approach was applied for the covariate selection. The final PopPK model, developed with data from 1293 patients ranging in age from 1 day to 18 years, was used to simulate enoxaparin subcutaneous dosing for prophylaxis and treatment based on total body weight (0-18 years, TBW) or fat-free mass (2-18 years, FFM). Simulated exposures in children with obesity (body mass index percentile ≥95th percentile) were compared with those without obesity.
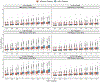
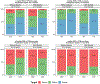
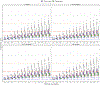
Results: A linear, one-compartment PopPK model that included allometric scaling using TBW (<2 years) or FFM (≥2 years) characterized the enoxaparin pharmacokinetic data. In addition, serum creatinine was identified as a significant covariate influencing clearance. Simulations indicated that in patients aged <2 years, the recommended 1.5 mg/kg TBW-based dosing achieves therapeutic simulated concentrations. In pediatric patients aged ≥2 years, the recommended 1.0 mg/kg dose resulted in exposures more comparable in children with and without obesity when FFM weight-based dosing was applied.
Conclusion: Using real-world data and PopPK modeling, enoxaparin's pharmacokinetics were characterized in pediatric patients. Using FFM and twice-daily dosing might reduce the risk of overdosing, especially in children with obesity.
© 2024. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Conflicts of Interest
V.E.H.’s affiliation with GSK did not present any conflict of interest regarding the research, findings, or conclusions presented in this manuscript. The other authors have no relevant conflicts of interest to disclose. The work conducted and reported herein is free from any financial or personal relationships that could potentially bias the results or interpretations.
Figures
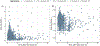

References
-
- Lovenox [product monograph] [Internet]. 2018. Available from: https://pdf.hres.ca/dpd_pm/00047708.PDF
-
- Schobess R, Düring C, Bidlingmaier C, Heinecke A, Merkel N, Nowak-Göttl U. Long-term safety and efficacy data on childhood venous thrombosis treated with a low molecular weight heparin: An open-label pilot study of once-daily versus twice-daily enoxaparin administration. Haematologica. 2006;91:1701–4. - PubMed
-
- Dix D, Andrew M, Marzinotto V, Charpentier K, Bridge S, Monagle P, et al. The use of low molecular weight heparin in pediatric patients: A prospective cohort study. J Pediatr. 2000;136:439–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

